Induction of ATF3 gene network by triglyceride-rich lipoprotein lipolysis products increases vascular apoptosis and inflammation
- PMID: 23868936
- PMCID: PMC3921073
- DOI: 10.1161/ATVBAHA.113.301375
Induction of ATF3 gene network by triglyceride-rich lipoprotein lipolysis products increases vascular apoptosis and inflammation
Abstract
Objective: Elevation of triglyceride-rich lipoproteins (TGRLs) contributes to the risk of atherosclerotic cardiovascular disease. Our work has shown that TGRL lipolysis products in high physiological to pathophysiological concentrations cause endothelial cell injury; however, the mechanisms remain to be delineated.
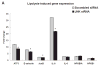
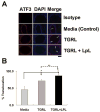
Approach and results: We analyzed the transcriptional signaling networks in arterial endothelial cells exposed to TGRL lipolysis products. When human aortic endothelial cells in culture were exposed to TGRL lipolysis products, activating transcription factor 3 (ATF3) was identified as a principal response gene. Induction of ATF3 mRNA and protein was confirmed by quantitative reverse-transcription polymerase chain reaction and Western blot respectively. Immunofluorescence analysis showed that ATF3 accumulated in the nuclei of cells treated with lipolysis products. Nuclear expression of phosphorylated c-Jun N-terminal kinase (JNK), previously shown to be an initiator of the ATF3 signaling cascade, also was demonstrated. Small interfering RNA (siRNA)-mediated inhibition of ATF3 blocked lipolysis products-induced transcription of E-selectin and interleukin-8, but not interleukin-6 or nuclear factor-κB. c-Jun, a downstream protein in the JNK pathway, was phosphorylated, whereas expression of nuclear factor-κB-dependent JunB was downregulated. Additionally, JNK siRNA suppressed ATF3 and p-c-Jun protein expression, suggesting that JNK is upstream of the ATF3 signaling pathway. In vivo studies demonstrated that infusion of TGRL lipolysis products into wild-type mice induced nuclear ATF3 accumulation in carotid artery endothelium. ATF3(-/-) mice were resistant to vascular apoptosis precipitated by treatment with TGRL lipolysis products. Also peripheral blood monocytes isolated from postprandial humans had increased ATF3 expression as compared with fasting monocytes.
Conclusions: This study demonstrates that TGRL lipolysis products activate ATF3-JNK transcription factor networks and induce endothelial cells inflammatory response.
Keywords: activating transcription factor 3; inflammation; lipolysis; lipoproteins; oligonucleotide arrays.
Figures

















References
-
- Norata GD, Grigore L, Raselli S, Seccomandi PM, Hamsten A, Maggi FM, Eriksson P, Catapano AL. Triglyceride-rich lipoproteins from hypertriglyceridemic subjects induce a pro-inflammatory response in the endothelium: Molecular mechanisms and gene expression studies. Journal of molecular and cellular cardiology. 2006;40(4):484–494. - PubMed
-
- Norata GD, Grigore L, Raselli S, Redaelli L, Hamsten A, Maggi F, Eriksson P, Catapano AL. Post-prandial endothelial dysfunction in hypertriglyceridemic subjects: molecular mechanisms and gene expression studies. Atherosclerosis. 2007;193(2):321–327. - PubMed
-
- Ting HJ, Stice JP, Schaff UY, Hui DY, Rutledge JC, Knowlton AA, Passerini AG, Simon SI. Triglyceride-rich lipoproteins prime aortic endothelium for an enhanced inflammatory response to tumor necrosis factor-alpha. Circulation research. 2007;100(3):381–390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

