Src mediates the mechanical activation of myogenesis by activating TNFα-converting enzyme
- PMID: 23868980
- PMCID: PMC3784819
- DOI: 10.1242/jcs.125328
Src mediates the mechanical activation of myogenesis by activating TNFα-converting enzyme
Abstract
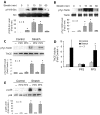
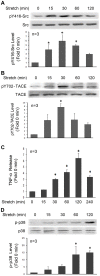
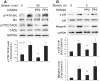
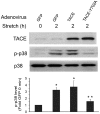
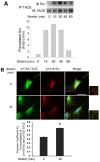
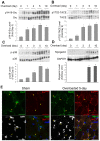
Mechanical stimulation affects many biological aspects in living cells through mechanotransduction. In myogenic precursor cells (MPCs), mechanical stimulation activates p38 mitogen-activated protein kinase (MAPK), a key regulator of myogenesis, via activating TNFα-converting enzyme (TACE, also known as ADAM17), to release autocrine TNFα. However, the signaling mechanism of mechanical activation of TACE is unknown. Because TACE possesses the structural features of substrates of the non-receptor tyrosine kinase Src, we tested the hypothesis that Src mediates mechanical activation of TACE in MPCs. We observed that mechanical stretch of C2C12 or primary rat myoblasts rapidly activates Src, which in turn interacts and colocalizes with TACE, resulting in tyrosine phosphorylation and activation of TACE. Particularly, Src activates TACE via the phosphorylation of amino acid residue Tyr702 in the intracellular tail of TACE, resulting in increased TNFα release and p38 activation. Src inhibition or deficiency blocks stretch activation of the TACE-p38-MAPK signaling, resulting in impaired myogenic gene expression. In response to functional overloading, Src and TACE are activated in mouse soleus muscle. Further, overloading-induced myogenesis and regeneration are impaired in the soleus of Src(+/-) mice. Therefore, Src mediates mechano-activation of TACE and myogenesis.
Keywords: ADAM17; Mechanotransduction; Muscle regeneration; Myogenic gene expression; Src; TACE; p38 MAPK.
Figures


Similar articles
-
Regulation of myogenic activation of p38 MAPK by TACE-mediated TNFα release.Front Cell Dev Biol. 2014 May 23;2:21. doi: 10.3389/fcell.2014.00021. eCollection 2014. Front Cell Dev Biol. 2014. PMID: 25364728 Free PMC article. Review.
-
TACE release of TNF-alpha mediates mechanotransduction-induced activation of p38 MAPK and myogenesis.J Cell Sci. 2007 Feb 15;120(Pt 4):692-701. doi: 10.1242/jcs.03372. Epub 2007 Jan 30. J Cell Sci. 2007. PMID: 17264149 Free PMC article.
-
TNFα shedding in mechanically stressed cardiomyocytes is mediated by Src activation of TACE.J Cell Biochem. 2015 Apr;116(4):559-65. doi: 10.1002/jcb.25006. J Cell Biochem. 2015. PMID: 25371038
-
TNF-alpha regulates myogenesis and muscle regeneration by activating p38 MAPK.Am J Physiol Cell Physiol. 2007 May;292(5):C1660-71. doi: 10.1152/ajpcell.00486.2006. Epub 2006 Dec 6. Am J Physiol Cell Physiol. 2007. PMID: 17151142 Free PMC article.
-
p38 MAPKs - roles in skeletal muscle physiology, disease mechanisms, and as potential therapeutic targets.JCI Insight. 2021 Jun 22;6(12):e149915. doi: 10.1172/jci.insight.149915. JCI Insight. 2021. PMID: 34156029 Free PMC article. Review.
Cited by
-
Exercise training and return to a well-balanced diet activate the neuregulin 1/ErbB pathway in skeletal muscle of obese rats.J Physiol. 2015 Jun 15;593(12):2665-77. doi: 10.1113/JP270026. Epub 2015 May 14. J Physiol. 2015. PMID: 25820551 Free PMC article.
-
Integrated Analysis Reveals a lncRNA-miRNA-mRNA Network Associated with Pigeon Skeletal Muscle Development.Genes (Basel). 2021 Nov 11;12(11):1787. doi: 10.3390/genes12111787. Genes (Basel). 2021. PMID: 34828393 Free PMC article.
-
Integrin and Its Associated Proteins as a Mediator for Mechano-Signal Transduction.Biomolecules. 2025 Jan 23;15(2):166. doi: 10.3390/biom15020166. Biomolecules. 2025. PMID: 40001469 Free PMC article. Review.
-
Regulation of myogenic activation of p38 MAPK by TACE-mediated TNFα release.Front Cell Dev Biol. 2014 May 23;2:21. doi: 10.3389/fcell.2014.00021. eCollection 2014. Front Cell Dev Biol. 2014. PMID: 25364728 Free PMC article. Review.
-
Myostatin/Activin Receptor Ligands in Muscle and the Development Status of Attenuating Drugs.Endocr Rev. 2022 Mar 9;43(2):329-365. doi: 10.1210/endrev/bnab030. Endocr Rev. 2022. PMID: 34520530 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

