Enantioconvergent cross-couplings of racemic alkylmetal reagents with unactivated secondary alkyl electrophiles: catalytic asymmetric Negishi α-alkylations of N-Boc-pyrrolidine
- PMID: 23869442
- PMCID: PMC3803154
- DOI: 10.1021/ja4054114
Enantioconvergent cross-couplings of racemic alkylmetal reagents with unactivated secondary alkyl electrophiles: catalytic asymmetric Negishi α-alkylations of N-Boc-pyrrolidine
Abstract
Although enantioconvergent alkyl-alkyl couplings of racemic electrophiles have been developed, there have been no reports of the corresponding reactions of racemic nucleophiles. Herein we describe Negishi cross-couplings of racemic α-zincated N-Boc-pyrrolidine with unactivated secondary halides, thus providing a one-pot, catalytic asymmetric method for the synthesis of a range of 2-alkylpyrrolidines (an important family of target molecules) from N-Boc-pyrrolidine, a commercially available precursor. Preliminary mechanistic studies indicated that two of the most straightforward mechanisms for enantioconvergence (dynamic kinetic resolution of the organometallic coupling partner and a simple β-hydride elimination/β-migratory insertion pathway) are unlikely to be operative.
Figures
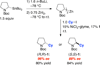

References
-
-
(a) For a recent report of a nickel-catalyzed process, see: Wilsily A, Tramutola F, Owston NA, Fu GC. J. Am. Chem. Soc. 2012;134:5794–5797. (b) For an overview of palladium-catalyzed couplings, see: Netherton MR, Fu GC. Top. Organomet. Chem. 2005;14:85–108.
-
-
-
For leading references, see: Glasspoole BW, Crudden CM. Nature Chem. 2011;3:912–913. Glorius F. Angew. Chem. Int. Ed. 2008;47:8347–8349. Rudolph A, Lautens M. Angew. Chem. Int. Ed. 2009;48:2656–2670. Jana R, Pathak TP, Sigman MS. Chem. Rev. 2011;111:1417–1492.
-
-
-
For examples of applications of palladium-catalyzed alkyl-alkyl cross-couplings in the total synthesis of natural products, see: Griggs ND, Phillips AJ. Org. Lett. 2008;10:4955–4957. Keaton KA, Phillips AJ. Org. Lett. 9:2007. 2717–2719.
-
-
-
(a) For a recent report of a Suzuki reaction, see Reference . (b) For an example of a Negishi reaction, see: Son S, Fu GC. J. Am. Chem. Soc. 2008;130:2756–2757.
-
-
-
For a recent application in the total synthesis of a natural product, see: Schmidt T, Kirschning A. Angew. Chem. Int. Ed. 2012;51:1063–1066.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

