Stem cells, cell therapies, and bioengineering in lung biology and diseases. Comprehensive review of the recent literature 2010-2012
- PMID: 23869446
- PMCID: PMC3960908
- DOI: 10.1513/AnnalsATS.201304-090AW
Stem cells, cell therapies, and bioengineering in lung biology and diseases. Comprehensive review of the recent literature 2010-2012
Abstract
A conference, "Stem Cells and Cell Therapies in Lung Biology and Lung Diseases," was held July 25 to 28, 2011 at the University of Vermont to review the current understanding of the role of stem and progenitor cells in lung repair after injury and to review the current status of cell therapy and ex vivo bioengineering approaches for lung diseases. These are rapidly expanding areas of study that provide further insight into and challenge traditional views of mechanisms of lung repair after injury and pathogenesis of several lung diseases. The goals of the conference were to summarize the current state of the field, to discuss and debate current controversies, and to identify future research directions and opportunities for basic and translational research in cell-based therapies for lung diseases. The goal of this article, which accompanies the formal conference report, is to provide a comprehensive review of the published literature in lung regenerative medicine from the last conference report through December 2012.
Figures



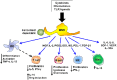
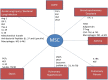
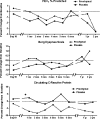
References
-
- Weiss DJ, Berberich MA, Borok Z, Gail DB, Kolls JK, Penland C, Prockop DJ. Adult stem cells, lung biology, and lung disease. NHLBI/Cystic Fibrosis Foundation Workshop. Proc Am Thorac Soc. 2006;3:193–207. - PubMed
-
- Brown JK, Hogan BLM, Randell SH, Stripp B, Weiss DJ. Human embryonic stem cell research: an official ATS research policy statement. Am J Respir Crit Care Med. 2006;173:1–3.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

