Temporal changes and regional differences in treatment uptake of hepatitis C therapy in EuroSIDA
- PMID: 23869664
- PMCID: PMC4030620
- DOI: 10.1111/hiv.12068
Temporal changes and regional differences in treatment uptake of hepatitis C therapy in EuroSIDA
Abstract
Objectives: All HIV/hepatitis C virus (HCV)-coinfected patients with chronic HCV infection and ≥ F2 fibrosis should be considered for HCV therapy. This study aimed to determine the rate of HCV treatment uptake among coinfected patients in Europe.
Methods: EuroSIDA patients with viraemic HCV infection were included in the study. Poisson regression was used to identify temporal changes and regional differences in HCV treatment uptake.
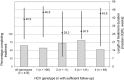
Results: A total of 1984 patients were included in the study, with a median follow-up time of 168 months [interquartile range (IQR) 121-204 months]. To date, 501 (25.3%) HIV/HCV-coinfected patients have received HCV therapy. Treatment incidence rose from 0.33 [95% confidence interval (CI) 0.16-0.50] per 100 person-years of follow-up (PYFU) in 1998 to 5.93 (95% CI 4.49-7.38) in 2007, falling to 3.78 (95% CI 2.50-5.07) in 2009. After adjustment, CD4 cell count > 350 cells/μL [incidence rate ratio (IRR) 1.33 (95% CI 1.06-1.67) vs. CD4 count 200-350 cells/μL] and ≥F2 liver fibrosis [IRR 1.60 (95% CI 1.14-2.25; P = 0.0065) vs. < F2 fibrosis] were predictors of anti-HCV treatment initiation. However, 22% of patients who remain untreated for HCV, with fibrosis data available, had ≥F2 fibrosis and should have been considered for treatment, while only 36% of treated patients had ≥F2 fibrosis.
Conclusions: Although treatment incidence for HCV has increased, there remain a large proportion of patients indicated for treatment who have yet to be treated.
Keywords: EuroSIDA; HIV/HCV coinfection; PEG-interferon; ribavirin; treatment completion.
© 2013 British HIV Association.
Figures

References
-
- Mocroft A, Reiss P, Gasiorowski J, et al. Serious fatal and nonfatal non-AIDS-defining illnesses in Europe. Jaids-J Acquir Immune Defic Syndr. 2010;55:262–270. - PubMed
-
- Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D: A: D study. Arch Intern Med. 2006;166:1632–1641. - PubMed
-
- Smit C, van den Berg C, Geskus R, Berkhout B, Coutinho R, Prins M. Risk of hepatitis-related mortality increased among hepatitis C virus/HIV-coinfected drug users compared with drug users infected only with hepatitis C virus – a 20-year prospective study. Jaids-J Acquir Immune Defic Syndr. 2008;47:221–225. - PubMed
-
- Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562–569. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

