TNFα-activated stromal COX-2 signalling promotes proliferative and invasive potential of colon cancer epithelial cells
- PMID: 23869759
- PMCID: PMC6495503
- DOI: 10.1111/cpr.12047
TNFα-activated stromal COX-2 signalling promotes proliferative and invasive potential of colon cancer epithelial cells
Abstract
Objectives: Up to now it has been unclear whether stromal/epithelial interaction affects progression of colon cancer. This study was designed to examine effects of tumour necrosis factor alpha (TNFα)-activated stromal cyclooxygenase-2 (COX-2) signalling on proliferation and invasiveness of colon cancer epithelial cells.
Materials and methods: Cyclooxygenase-2 mRNA and protein were determined by real-time PCR and western blotting and prostaglandin E2 (PGE2 ) was assayed by radioimmunoassay. Cell proliferation and invasiveness were determined by transwell chamber assays and protein kinase C (PKC) was assayed by Biotrak(™) PKC Assay System.
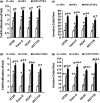
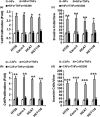
Results: Our results indicated that TNFα, a powerful inflammatory cytokine, strongly promoted COX-2 expression and PGE2 production in colon cancer-associated fibroblasts. Using in vitro assays for estimating proliferative and invasive potential, we discovered that activation of stromal COX-2 signalling significantly promoted proliferation and invasiveness of colon cancer epithelial cells. In addition, selective COX-2 inhibitor N-[2-(Cyclohexyloxy)-4-nitrophenyl]methanesulfonamide, blocked such proliferative and invasive effects on the cancer epithelial cells. In this process, PKC was involved in activation of COX-2 signalling in the fibroblasts.
Conclusion: We conclude that activation of stromal COX-2 signalling by TNFα played a major role in promoting proliferation and invasiveness of colon cancer epithelial cells.
© 2013 John Wiley & Sons Ltd.
Figures


Similar articles
-
IL1β-mediated Stromal COX-2 signaling mediates proliferation and invasiveness of colonic epithelial cancer cells.Exp Cell Res. 2012 Nov 15;318(19):2520-30. doi: 10.1016/j.yexcr.2012.07.021. Epub 2012 Aug 4. Exp Cell Res. 2012. PMID: 22884582
-
HGF-activated colonic fibroblasts mediates carcinogenesis of colonic epithelial cancer cells via PKC-cMET-ERK1/2-COX-2 signaling.Cell Signal. 2015 Apr;27(4):860-6. doi: 10.1016/j.cellsig.2015.01.014. Epub 2015 Jan 30. Cell Signal. 2015. PMID: 25643632
-
Stromal COX-2 signaling activated by deoxycholic acid mediates proliferation and invasiveness of colorectal epithelial cancer cells.Biochem Biophys Res Commun. 2012 Aug 31;425(3):607-12. doi: 10.1016/j.bbrc.2012.07.137. Epub 2012 Aug 2. Biochem Biophys Res Commun. 2012. PMID: 22885178
-
The COX-2 selective inhibitor-independent COX-2 effect on colon carcinoma cells is associated with the Delta1/Notch1 pathway.Dig Dis Sci. 2008 Aug;53(8):2195-203. doi: 10.1007/s10620-007-0139-0. Epub 2008 Mar 5. Dig Dis Sci. 2008. PMID: 18320325
-
Pharmacological inhibition of platelet-tumor cell cross-talk prevents platelet-induced overexpression of cyclooxygenase-2 in HT29 human colon carcinoma cells.Mol Pharmacol. 2013 Jul;84(1):25-40. doi: 10.1124/mol.113.084988. Epub 2013 Apr 11. Mol Pharmacol. 2013. PMID: 23580446 Free PMC article.
Cited by
-
Glyburide Suppresses Inflammation-Related Colorectal Tumorigenesis Through Inhibition of NLRP3 Inflammasome.Int J Mol Sci. 2024 Oct 30;25(21):11640. doi: 10.3390/ijms252111640. Int J Mol Sci. 2024. PMID: 39519191 Free PMC article.
-
Dual COX-2/TNF-α Inhibitors as Promising Anti-inflammatory and Cancer Chemopreventive Agents: A Review.Iran J Pharm Res. 2024 Oct 29;23(1):e151312. doi: 10.5812/ijpr-151312. eCollection 2024 Jan-Dec. Iran J Pharm Res. 2024. PMID: 39830670 Free PMC article. Review.
-
RNF186/EPHB2 Axis Is Essential in Regulating TNF Signaling for Colorectal Tumorigenesis in Colorectal Epithelial Cells.J Immunol. 2022 Nov 1;209(9):1796-1805. doi: 10.4049/jimmunol.2200229. Epub 2022 Sep 21. J Immunol. 2022. PMID: 36130827 Free PMC article.
-
Jacalin Has Chemopreventive Effects on Colon Cancer Development.Biomed Res Int. 2017;2017:4614357. doi: 10.1155/2017/4614357. Epub 2017 Jun 6. Biomed Res Int. 2017. PMID: 28676858 Free PMC article.
-
Fibroblast Subsets in Intestinal Homeostasis, Carcinogenesis, Tumor Progression, and Metastasis.Cancers (Basel). 2021 Jan 7;13(2):183. doi: 10.3390/cancers13020183. Cancers (Basel). 2021. PMID: 33430285 Free PMC article. Review.
References
-
- Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J. Clin. 60, 277–300. - PubMed
-
- Kargman SL, O'Neill GP, Vickers PJ, Evans JF, Mancini JA, Jothy S (1995) Expression of prostaglandin G/H synthase‐1 and ‐2 protein in human colon cancer. Cancer Res. 55, 2556–2559. - PubMed
-
- Fodde R (2002) The APC gene in colorectal cancer. Eur. J. Cancer 38, 867–871. - PubMed
-
- Mueller MM, Fusenig NE (2004) Friends or foes – bipolar effects of the tumour stroma in cancer. Nat. Rev. Cancer 4, 839–849. - PubMed
-
- Zhu Y, Hua P, Lance P (2003) Cyclooxygenase‐2 expression and prostanoid biogenesis reflect clinical phenotype in human colorectal fibroblast strains. Cancer Res. 63, 522–526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

