Role of oxidative stress and the microenvironment in breast cancer development and progression
- PMID: 23870510
- PMCID: PMC3950899
- DOI: 10.1016/B978-0-12-407190-2.00003-4
Role of oxidative stress and the microenvironment in breast cancer development and progression
Abstract
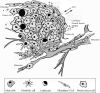
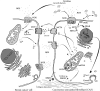
Breast cancer is a highly complex tissue composed of neoplastic and stromal cells. Carcinoma-associated fibroblasts (CAFs) are commonly found in the cancer stroma, where they promote tumor growth and enhance vascularity in the microenvironment. Upon exposure to oxidative stress, fibroblasts undergo activation to become myofibroblasts. These cells are highly mobile and contractile and often express numerous mesenchymal markers. CAF activation is irreversible, making them incapable of being removed by nemosis. In breast cancer, almost 80% of stromal fibroblasts acquire an activated phenotype that manifests by secretion of elevated levels of growth factors, cytokines, and metalloproteinases. They also produce hydrogen peroxide, which induces the generation of subsequent sets of activated fibroblasts and tumorigenic alterations in epithelial cells. While under oxidative stress, the tumor stroma releases high energy nutrients that fuel cancer cells and facilitate their growth and survival. This review describes how breast cancer progression is dependent upon oxidative stress activated stroma and proposes potential new therapeutic avenues.
Keywords: Breast cancer; Cancer-associated fibroblasts; Microenvironment; Myofibroblasts; Oxidative stress; Reactive oxygen species; Stroma-associated fibroblasts.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

References
-
- Boidot R, et al. Regulation of monocarboxylate transporter MCT1 expression by p53 mediates inward and outward lactate fluxes in tumors. Cancer Research. 2012;72(4):939–948. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

