A multicenter evaluation of oral pressure therapy for the treatment of obstructive sleep apnea
- PMID: 23871259
- PMCID: PMC3932027
- DOI: 10.1016/j.sleep.2013.05.009
A multicenter evaluation of oral pressure therapy for the treatment of obstructive sleep apnea
Abstract
Objective: We aimed to evaluate the impact of a novel noninvasive oral pressure therapy (OPT) (Winx®, ApniCure) system on polysomnographic measures of sleep-disordered breathing, sleep architecture, and sleep stability in obstructive sleep apnea (OSA).
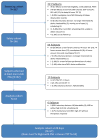
Subjects and methods: A 4-week, multicenter, prospective, open-label, randomized, crossover, first-night order of control vs treatment, single-arm trial was conducted in five American Academy of Sleep Medicine (AASM) - accredited sleep clinics and one research laboratory. Sixty-three subjects (analysis cohort) were studied from a screening cohort of 367 subjects. The analysis cohort was 69.8% men, ages 53.6±8.9 years (mean±SD), body mass index of 32.3±4.5kg/m(2), with mild to severe OSA. At treatment initiation, subjects received random assignment to one night with and one without (control) treatment, and they were assessed again following 28 nights of treatment. Breathing and sleep architecture were assessed each night based on blind scoring by a single centralized scorer using AASM criteria.
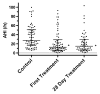
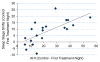
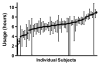
Results: Average nightly usage across the take-home period was 6.0±1.4h. There were no severe or serious device-related adverse events (AEs). Median apnea-hypopnea index (AHI) was 27.5 events per hour on the control night, 13.4 events per hour on the first treatment night, and 14.8 events per hour after 28days of treatment. A clinically significant response (treatment AHI ⩽10/h and ⩽50% of control values) was seen in 20 of the 63 subjects evaluated. Rapid eye movement percentage (REM%) was significantly increased, and N1%, stage shifts to N1 sleep, overall stage shifts, total awakenings, and arousals per hour were all significantly reduced at both treatment nights compared to controls. Mean Epworth sleepiness scale (ESS) was significantly reduced from 12.1 to 8.6 (Cohen d effect size, 0.68) in those untreated for two or more weeks prior to OPT study participation and remained unchanged in subjects who directly switched from continuous positive airway pressure (CPAP) therapy to OPT.
Conclusion: Clinically significant improvements in sleep quality and continuity, AHI, ODI, ESS, and overall clinical status were achieved in an easily identified subgroup. OPT was safe and well-tolerated and nightly usage was high.
Keywords: AHI; Compliance; Epworth sleepiness scale; ODI; Obstructive sleep apnea; Oral pressure therapy.
Copyright © 2013 Elsevier B.V. All rights reserved.
Conflict of interest statement
Dr Black is a consultant to ApniCure and Dr Siegel is employed by ApniCure.
Figures


Similar articles
-
A feasibility evaluation of oral pressure therapy for the treatment of obstructive sleep apnea.Ther Adv Respir Dis. 2013 Feb;7(1):3-12. doi: 10.1177/1753465812468043. Epub 2012 Nov 26. Ther Adv Respir Dis. 2013. PMID: 23184709 Clinical Trial.
-
Effect of Multilevel Upper Airway Surgery vs Medical Management on the Apnea-Hypopnea Index and Patient-Reported Daytime Sleepiness Among Patients With Moderate or Severe Obstructive Sleep Apnea: The SAMS Randomized Clinical Trial.JAMA. 2020 Sep 22;324(12):1168-1179. doi: 10.1001/jama.2020.14265. JAMA. 2020. PMID: 32886102 Free PMC article. Clinical Trial.
-
A multisite randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: the HomePAP study.Sleep. 2012 Jun 1;35(6):757-67. doi: 10.5665/sleep.1870. Sleep. 2012. PMID: 22654195 Free PMC article. Clinical Trial.
-
The comparison of CPAP and oral appliances in treatment of patients with OSA: a systematic review and meta-analysis.Respir Care. 2013 Jul;58(7):1184-95. doi: 10.4187/respcare.02245. Epub 2013 Jan 3. Respir Care. 2013. PMID: 23287015
-
Sleep apnea is a common and dangerous cardiovascular risk factor.Curr Probl Cardiol. 2025 Jan;50(1):102838. doi: 10.1016/j.cpcardiol.2024.102838. Epub 2024 Sep 4. Curr Probl Cardiol. 2025. PMID: 39242062 Review.
Cited by
-
Obstructive Sleep Apnea Screening in Patients With Atrial Fibrillation: Missed Opportunities for Early Diagnosis.J Clin Med Res. 2019 Jan;11(1):21-25. doi: 10.14740/jocmr3635. Epub 2018 Dec 3. J Clin Med Res. 2019. PMID: 30627274 Free PMC article.
-
Improvements in current treatments and emerging therapies for adult obstructive sleep apnea.F1000Prime Rep. 2014 May 6;6:36. doi: 10.12703/P6-36. eCollection 2014. F1000Prime Rep. 2014. PMID: 24860658 Free PMC article. Review.
-
Alternative approaches to treatment of Central Sleep Apnea.Sleep Med Clin. 2014 Mar 1;9(1):87-104. doi: 10.1016/j.jsmc.2013.10.008. Sleep Med Clin. 2014. PMID: 24772053 Free PMC article.
-
New developments in the use of positive airway pressure for obstructive sleep apnea.J Thorac Dis. 2015 Aug;7(8):1323-42. doi: 10.3978/j.issn.2072-1439.2015.07.30. J Thorac Dis. 2015. PMID: 26380760 Free PMC article. Review.
-
A Young Man Running Out of Treatment Options.J Clin Sleep Med. 2015 Oct 15;11(10):1239-42. doi: 10.5664/jcsm.5104. J Clin Sleep Med. 2015. PMID: 26156953 Free PMC article. No abstract available.
References
-
- Redline S, Young T. Epidemiology and natural history of obstructive sleep apnea. Ear Nose Throat J. 1993;72(20–1):4–6. - PubMed
-
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. - PubMed
-
- Guilleminault C, Eldridge FL, Tilkian A, Simmons FB, Dement WC. Sleep apnea syndrome due to upper airway obstruction: a review of 25 cases. Arch Intern Med. 1977;137:296–300. - PubMed
-
- Harris M, Glozier N, Ratnavadivel R, Grunstein RR. Obstructive sleep apnea and depression. Sleep Med Rev. 2009;13:437–44. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

