A core human primary tumor angiogenesis signature identifies the endothelial orphan receptor ELTD1 as a key regulator of angiogenesis
- PMID: 23871637
- PMCID: PMC3743050
- DOI: 10.1016/j.ccr.2013.06.004
A core human primary tumor angiogenesis signature identifies the endothelial orphan receptor ELTD1 as a key regulator of angiogenesis
Abstract
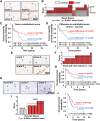
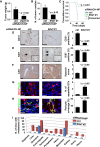
Limited clinical benefits derived from anti-VEGF therapy have driven the identification of new targets involved in tumor angiogenesis. Here, we report an integrative meta-analysis to define the transcriptional program underlying angiogenesis in human cancer. This approach identified ELTD1, an orphan G-protein-coupled receptor whose expression is induced by VEGF/bFGF and repressed by DLL4 signaling. Extensive analysis of multiple cancer types demonstrates significant upregulation of ELTD1 in tumor-associated endothelial cells, with a higher expression correlating with favorable prognosis. Importantly, ELTD1 silencing impairs endothelial sprouting and vessel formation in vitro and in vivo, drastically reducing tumor growth and greatly improving survival. Collectively, these results provide insight into the regulation of tumor angiogenesis and highlight ELTD1 as key player in blood vessel formation.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Bais, C., Singh, M., Kaminker, J., and Brauer, M. May 2011. Biological markers for monitoring patient response to vegf antagonists. U.S. Patent 20,110,117,083.
-
- Baldewijns M.M., van Vlodrop I.J., Vermeulen P.B., Soetekouw P.M., van Engeland M., de Bruïne A.P. VHL and HIF signalling in renal cell carcinogenesis. J. Pathol. 2010;221:125–138. - PubMed
-
- Bjarnadóttir T.K., Fredriksson R., Höglund P.J., Gloriam D.E., Lagerström M.C., Schiöth H.B. The human and mouse repertoire of the adhesion family of G-protein-coupled receptors. Genomics. 2004;84:23–33. - PubMed
-
- Bubendorf L., Nocito A., Moch H., Sauter G. Tissue microarray (TMA) technology: miniaturized pathology archives for high-throughput in situ studies. J. Pathol. 2001;195:72–79. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

