Maternal nutrition and risk of obesity in offspring: the Trojan horse of developmental plasticity
- PMID: 23871838
- PMCID: PMC3855628
- DOI: 10.1016/j.bbadis.2013.07.007
Maternal nutrition and risk of obesity in offspring: the Trojan horse of developmental plasticity
Abstract
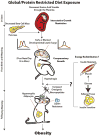
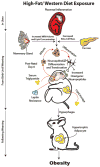
Mammalian embryos have evolved to adjust their organ and tissue development in response to an atypical environment. This adaptation, called phenotypic plasticity, allows the organism to thrive in the anticipated environment in which the fetus will emerge. Barker and colleagues proposed that if the environment in which the fetus emerges differs from that in which it develops, phenotypic plasticity may provide an underlying mechanism for disease. Epidemiological studies have shown that humans born small- or large-for-gestational-age, have a higher likelihood of developing obesity as adults. The amount and quality of food that the mother consumes during gestation influences birth weight, and therefore susceptibility of progeny to disease in later life. Studies in experimental animals support these observations, and find that obesity occurs as a result of maternal nutrient-restriction during gestation, followed by rapid compensatory growth associated with ad libitum food consumption. Therefore, obesity associated with maternal nutritional restriction has a developmental origin. Based on this phenomenon, one might predict that gestational exposure to a westernized diet would protect against future obesity in offspring. However, evidence from experimental models indicates that, like maternal dietary restriction, maternal consumption of a westernized diet during gestation and lactation interacts with an adult obesogenic diet to induce further obesity. Mechanistically, restriction of nutrients or consumption of a high fat diet during gestation may promote obesity in progeny by altering hypothalamic neuropeptide production and thereby increasing hyperphagia in offspring. In addition to changes in food intake these animals may also direct energy from muscle toward storage in adipose tissue. Surprisingly, generational inheritance studies in rodents have further indicated that effects on body length, body weight, and glucose tolerance appear to be propagated to subsequent generations. Together, the findings discussed herein highlight the concept that maternal nutrition contributes to a legacy of obesity. Thus, ensuring adequate supplies of a complete and balanced diet during and after pregnancy should be a priority for public health worldwide. This article is part of a Special Issue entitled: Modulation of Adipose Tissue in Health and Disease.
Keywords: Maternal health; Nutrition; Obesity.
© 2013.
Figures

References
-
- Koopman P. Organogenesis in development. Academic Press; San Diego, Calif: 2010. ScienceDirect.
-
- McCance RA. Food, growth, and time. Lancet. 1962;2:621–626. - PubMed
-
- Sasaki A, Nakagawa I, Kajimoto M. Effect of protein nutrition throughout gestation and lactation on growth, morbidity and life span of rat progeny. J Nutr Sci Vitaminol (Tokyo) 1982;28:543–555. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

