Protease-activated receptor-2 regulates the innate immune response to viral infection in a coxsackievirus B3-induced myocarditis
- PMID: 23871888
- PMCID: PMC4077621
- DOI: 10.1016/j.jacc.2013.05.076
Protease-activated receptor-2 regulates the innate immune response to viral infection in a coxsackievirus B3-induced myocarditis
Abstract
Objectives: This study sought to evaluate the role of protease-activated receptor-2 (PAR2) in coxsackievirus B3 (CVB3)-induced myocarditis.
Background: An infection with CVB3 leads to myocarditis. PAR2 modulates the innate immune response. Toll-like receptor-3 (TLR3) is crucial for the innate immune response by inducing the expression of the antiviral cytokine interferon-beta (IFNβ).
Methods: To induce myocarditis, wild-type (wt) and PAR2 knockout (ko) mice were infected with 10(5) plaque-forming units CVB3. Mice underwent hemodynamic measurements with a 1.2-F microconductance catheter. Wt and PAR2ko hearts and cardiac cells were analyzed for viral replication and immune response with plaque assay, quantitative polymerase chain reaction, Western blot, and immunohistochemistry.
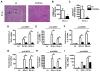
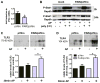
Results: Compared with wt mice, PAR2ko mice and cardiomyocytes exhibited a reduced viral load and developed no myocarditis after infection with CVB3. Hearts and cardiac fibroblasts from PAR2ko mice expressed higher basal levels of IFNβ than wt mice did. Treatment with CVB3 and polyinosinic:polycytidylic acid led to higher IFNβ expression in PAR2ko than in wt fibroblasts and reduced virus replication in PAR2ko fibroblasts was abrogated by neutralizing IFNβ antibody. Overexpression of PAR2 reduced the basal IFNβ expression. Moreover, a direct interaction between PAR2 and Toll-like receptor 3 was observed. PAR2 expression in endomyocardial biopsies of patients with nonischemic cardiomyopathy was positively correlated with myocardial inflammation and negatively with IFNβ expression and left ventricular ejection fraction.
Conclusions: PAR2 negatively regulates the innate immune response to CVB3 infection and contributes to myocardial dysfunction. The antagonism of PAR2 is of therapeutic interest to strengthen the antiviral response after an infection with a cardiotropic virus.
Keywords: AP; CAR; CCL; CD; CVB3; DAF; DMEM; Dulbecco modified Eagle medium; FBS; HL; IFNβ; IFNγ; IL; LVEF; P-Stat1; PAR2; PBS; PCR; TLR; TNFα; Toll-like receptor; Toll-like receptor 3; activating peptide; chemokine ligand; cluster of differentiation; coxsackievirus B3; coxsackievirus-adenovirus receptor; decay-accelerating receptor; double-stranded ribonucleic acid; dsRNA; fetal bovine serum; human leukocyte; interferon beta; interferon gamma; interferon-beta; interleukin; knockout; ko; left ventricular ejection fraction; mRNA; messenger ribonucleic acid; mock plasmid; myocarditis; p.i.; pVitro; phosphate-buffered saline; phospho-signal transducer and activator of transcription-1; poly(I:C); polyinosinic: polycytidylic acid; polymerase chain reaction; post-infection; protease-activated receptor 2; protease-activated receptor-2; tumor necrosis factor-alpha; wild type; wt.
Copyright © 2013 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures


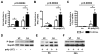

Comment in
-
Basic research on myocarditis: superb but unrequited.J Am Coll Cardiol. 2013 Nov 5;62(19):1746-7. doi: 10.1016/j.jacc.2013.06.030. Epub 2013 Jul 17. J Am Coll Cardiol. 2013. PMID: 23871883 No abstract available.
Similar articles
-
Adiponectin promotes coxsackievirus B3 myocarditis by suppression of acute anti-viral immune responses.Basic Res Cardiol. 2014 May;109(3):408. doi: 10.1007/s00395-014-0408-y. Epub 2014 Apr 2. Basic Res Cardiol. 2014. PMID: 24691762
-
Protein Kinase B2 (PKB2/AKT2) Is Essential for Host Protection in CVB3-Induced Acute Viral Myocarditis.Int J Mol Sci. 2022 Jan 27;23(3):1489. doi: 10.3390/ijms23031489. Int J Mol Sci. 2022. PMID: 35163412 Free PMC article.
-
Roles of PAR1 and PAR2 in viral myocarditis.Thromb Res. 2014 May;133 Suppl 1(0 1):S18-20. doi: 10.1016/j.thromres.2014.03.011. Thromb Res. 2014. PMID: 24759133 Free PMC article.
-
The Role of Protease-Activated Receptors for the Development of Myocarditis: Possible Therapeutic Implications.Curr Pharm Des. 2016;22(4):472-84. doi: 10.2174/1381612822666151222160933. Curr Pharm Des. 2016. PMID: 26696253 Review.
-
Multiple roles of the coagulation protease cascade during virus infection.Blood. 2014 Apr 24;123(17):2605-13. doi: 10.1182/blood-2013-09-526277. Epub 2014 Mar 14. Blood. 2014. PMID: 24632711 Free PMC article. Review.
Cited by
-
PAR2 Promoter Hypomethylation Regulates PAR2 Gene Expression and Promotes Lung Adenocarcinoma Cell Progression.Comput Math Methods Med. 2021 Apr 15;2021:5542485. doi: 10.1155/2021/5542485. eCollection 2021. Comput Math Methods Med. 2021. PMID: 33968158 Free PMC article.
-
Hypoxia-inducible transcription factor-1α inhibition by topotecan protects against lipopolysaccharide-induced inflammation and apoptosis of cardiomyocytes.Biomed Eng Online. 2021 Aug 31;20(1):88. doi: 10.1186/s12938-021-00923-2. Biomed Eng Online. 2021. PMID: 34465337 Free PMC article.
-
Protease-activated receptor 4 protects mice from Coxsackievirus B3 and H1N1 influenza A virus infection.Cell Immunol. 2019 Oct;344:103949. doi: 10.1016/j.cellimm.2019.103949. Epub 2019 Jul 3. Cell Immunol. 2019. PMID: 31337508 Free PMC article.
-
Cytotoxic CD8+ T Cells Are Involved in the Thrombo-Inflammatory Response during First-Diagnosed Atrial Fibrillation.Cells. 2022 Dec 29;12(1):141. doi: 10.3390/cells12010141. Cells. 2022. PMID: 36611934 Free PMC article.
-
MicroRNA-20b suppresses the expression of ZFP-148 in viral myocarditis.Mol Cell Biochem. 2017 May;429(1-2):199-210. doi: 10.1007/s11010-017-2947-7. Epub 2017 Feb 28. Mol Cell Biochem. 2017. Retraction in: Mol Cell Biochem. 2023 Jun;478(6):1413. doi: 10.1007/s11010-022-04598-8. PMID: 28247213 Retracted.
References
-
- Liu PP, Yan AT. Cardiovascular magnetic resonance for the diagnosis of acute myocarditis: prospects for detecting myocardial inflammation. J Am Coll Cardiol. 2005;45:1823–5. - PubMed
-
- Esfandiarei M, McManus BM. Molecular biology and pathogenesis of viral myocarditis. Annu Rev Pathol. 2008;3:127–55. - PubMed
-
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–8. - PubMed
-
- Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

