Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes
- PMID: 23872331
- PMCID: PMC3833827
- DOI: 10.1016/j.yfrne.2013.07.003
Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes
Abstract
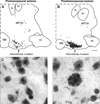
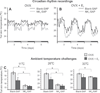
Despite affecting millions of individuals, the etiology of hot flushes remains unknown. Here we review the physiology of hot flushes, CNS pathways regulating heat-dissipation effectors, and effects of estrogen on thermoregulation in animal models. Based on the marked changes in hypothalamic kisspeptin, neurokinin B and dynorphin (KNDy) neurons in postmenopausal women, we hypothesize that KNDy neurons play a role in the mechanism of flushes. In the rat, KNDy neurons project to preoptic thermoregulatory areas that express the neurokinin 3 receptor (NK3R), the primary receptor for NKB. Furthermore, activation of NK₃R in the median preoptic nucleus, part of the heat-defense pathway, reduces body temperature. Finally, ablation of KNDy neurons reduces cutaneous vasodilatation and partially blocks the effects of estrogen on thermoregulation. These data suggest that arcuate KNDy neurons relay estrogen signals to preoptic structures regulating heat-dissipation effectors, supporting the hypothesis that KNDy neurons participate in the generation of flushes.
Keywords: Estrogen; GnRH; LH; Menopause; Reproduction; Thermoregulation.
Published by Elsevier Inc.
Figures










References
-
- Abel TW, Rance NE. Stereologic study of the hypothalamic infundibular nucleus in young and older women. J. Comp. Neurol. 2000;424:679–688. - PubMed
-
- Abel TW, Voytko ML, Rance NE. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. J. Clin. Endocrinol. Metab. 1999;84:2111–2118. - PubMed
-
- Alfinito PD, Huselton C, Chen X, Deecher DC. Pharmacokinetic and pharmacodynamic profiles of the novel serotonin and norepinephrine reuptake inhibitor desvenlafaxine succinate in ovariectomized Sprague-Dawley rats. Brain Res. 2006;1098:71–78. - PubMed
-
- Almeida MC, Steiner AA, Branco LG, Romanovsky AA. Cold-seeking behavior as a thermoregulatory strategy in systemic inflammation. Eur. J. Neurosci. 2006a;23:3359–3367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

