Effect of human 15-lipoxygenase-1 metabolites on vascular function in mouse mesenteric arteries and hearts
- PMID: 23872364
- PMCID: PMC3844054
- DOI: 10.1016/j.prostaglandins.2013.07.002
Effect of human 15-lipoxygenase-1 metabolites on vascular function in mouse mesenteric arteries and hearts
Abstract
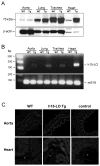
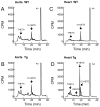
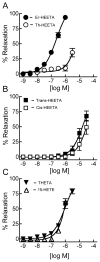
Lipoxygenases regulate vascular function by metabolizing arachidonic acid (AA) to dilator eicosanoids. Previously, we showed that endothelium-targeted adenoviral vector-mediated gene transfer of the human 15-lipoxygenase-1 (h15-LO-1) enhances arterial relaxation through the production of vasodilatory hydroxyepoxyeicosatrienoic acid (HEETA) and trihydroxyeicosatrienoic acid (THETA) metabolites. To further define this function, a transgenic (Tg) mouse line that overexpresses h15-LO-1 was studied. Western blot, immunohistochemistry and RT-PCR results confirmed expression of 15-LO-1 transgene in tissues, especially high quantity in coronary arterial wall, of Tg mice. Reverse-phase HPLC analysis of [(14)C]-AA metabolites in heart tissues revealed enhanced 15-HETE synthesis in Tg vs. WT mice. Among the 15-LO-1 metabolites, 15-HETE, erythro-13-H-14,15-EETA, and 11(R),12(S),15(S)-THETA relaxed the mouse mesenteric arteries to the greatest extent. The presence of h15-LO-1 increased acetylcholine- and AA-mediated relaxation in mesenteric arteries of Tg mice compared to WT mice. 15-LO-1 was most abundant in the heart; therefore, we used the Langendorff heart model to test the hypothesis that elevated 15-LO-1 levels would increase coronary flow following a short ischemia episode. Both peak flow and excess flow of reperfused hearts were significantly elevated in hearts from Tg compared to WT mice being 2.03 and 3.22 times greater, respectively. These results indicate that h15-LO-1-derived metabolites are highly vasoactive and may play a critical role in regulating coronary blood flow.
Keywords: 15-Lipoxygenase; AA; ACh; Coronary flow; EDHFs; Eicosanoids; Endothelium-derived hyperpolarizing factor; HEETAs; HETEs; HPETEs; Indo; Ischemia/reperfusion; LOs; NDGA; Reactive hyperemia; THETAs; Vasodilation; acetylcholine; arachidonic acid; endothelium-derived hyperpolarizing factors; hydroperoxyeicosatetraenoic acids; hydroxy-epoxyeicosatrienoic acids; indomethacin; l-NA; lipoxygenases; monohydroperoxyeicosatetraenoic acids; nitro-l-arginine; nordihydroguaiaretic acid; sEH; soluble epoxide hydrolase; trihydroxyeicosatrienoic acids.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
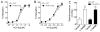

Similar articles
-
Role of arachidonic acid lipoxygenase metabolites in acetylcholine-induced relaxations of mouse arteries.Am J Physiol Heart Circ Physiol. 2011 Mar;300(3):H725-35. doi: 10.1152/ajpheart.00696.2009. Epub 2010 Dec 30. Am J Physiol Heart Circ Physiol. 2011. PMID: 21193584 Free PMC article.
-
15-Lipoxygenase metabolites contribute to age-related reduction in acetylcholine-induced hypotension in rabbits.Am J Physiol Heart Circ Physiol. 2008 Jul;295(1):H89-96. doi: 10.1152/ajpheart.00054.2008. Epub 2008 May 2. Am J Physiol Heart Circ Physiol. 2008. PMID: 18456739 Free PMC article.
-
Endothelial 15-lipoxygenase-1 overexpression increases acetylcholine-induced hypotension and vasorelaxation in rabbits.Hypertension. 2008 Feb;51(2):246-51. doi: 10.1161/HYPERTENSIONAHA.107.104125. Epub 2008 Jan 7. Hypertension. 2008. PMID: 18180398
-
Role of arachidonic acid lipoxygenase metabolites in the regulation of vascular tone.Am J Physiol Heart Circ Physiol. 2009 Aug;297(2):H495-507. doi: 10.1152/ajpheart.00349.2009. Epub 2009 Jun 12. Am J Physiol Heart Circ Physiol. 2009. PMID: 19525377 Free PMC article. Review.
-
Inducible endothelium-derived hyperpolarizing factor: role of the 15-lipoxygenase-EDHF pathway.J Cardiovasc Pharmacol. 2013 Mar;61(3):176-87. doi: 10.1097/FJC.0b013e31828165db. J Cardiovasc Pharmacol. 2013. PMID: 23249676 Free PMC article. Review.
Cited by
-
(5Z,11Z,15R)-15-Hydroxyeicosa-5,11-dien-13-ynoic acid: A stable isomer of 15(S)-HETE that retains key vasoconstrictive and antiproliferative activity.Prostaglandins Other Lipid Mediat. 2016 Mar;123:33-9. doi: 10.1016/j.prostaglandins.2016.04.001. Epub 2016 Apr 23. Prostaglandins Other Lipid Mediat. 2016. PMID: 27117058 Free PMC article.
-
Overview of Antagonists Used for Determining the Mechanisms of Action Employed by Potential Vasodilators with Their Suggested Signaling Pathways.Molecules. 2016 Apr 15;21(4):495. doi: 10.3390/molecules21040495. Molecules. 2016. PMID: 27092479 Free PMC article. Review.
-
Protection from hypertension in mice by the Mediterranean diet is mediated by nitro fatty acid inhibition of soluble epoxide hydrolase.Proc Natl Acad Sci U S A. 2014 Jun 3;111(22):8167-72. doi: 10.1073/pnas.1402965111. Epub 2014 May 19. Proc Natl Acad Sci U S A. 2014. PMID: 24843165 Free PMC article.
-
Restoration of miR-223-3p expression in aged mouse uteri with Samul-tang administration.Integr Med Res. 2022 Jun;11(2):100835. doi: 10.1016/j.imr.2022.100835. Epub 2022 Jan 24. Integr Med Res. 2022. PMID: 35141134 Free PMC article.
-
Inhibition of soluble epoxide hydrolase increases coronary perfusion in mice.Physiol Rep. 2015 Jun;3(6):e12427. doi: 10.14814/phy2.12427. Physiol Rep. 2015. PMID: 26071213 Free PMC article.
References
-
- Pfister SL, Spitzbarth N, Nithipatikom K, Edgemond WS, Falck JR, Campbell WB. Identification of 11,14,15- and 11,12,15-trihydroxyeicosatrienoic acids as endothelium-derived relaxing factors of rabbit aorta. J Biol Chem. 1998;273:30879–87. - PubMed
-
- Tang X, Holmes BB, Nithipatikom K, Hillard CJ, Kuhn H, Campbell WB. Reticulocyte 15-lipoxygenase-1 is important in acetylcholine-induced endothelium-dependent vasorelaxation in rabbit aorta. Arterioscler Thromb Vasc Biol. 2006;26:78–84. - PubMed
-
- Campbell WB, Spitzbarth N, Gauthier KM, Pfister SL. 11,12,15-Trihydroxyeicosatrienoic acid mediates acetylcholine-induced relaxations in the rabbit aorta. Am J Physiol. 2003;285:H2648–H56. - PubMed
-
- Gauthier KM, Spitzbarth N, Edwards EM, Campbell WB. Apamin-sensitive K+ currents mediate arachidonic acid-induced relaxations of rabbit aorta. Hypertension. 2004;43:413–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

