Muscle wasting from kidney failure-a model for catabolic conditions
- PMID: 23872437
- PMCID: PMC3919551
- DOI: 10.1016/j.biocel.2013.06.027
Muscle wasting from kidney failure-a model for catabolic conditions
Abstract
Purpose: Muscle atrophy is a frequent complication of chronic kidney disease (CKD) and is associated with increased morbidity and mortality. The processes causing loss of muscle mass are also present in several catabolic conditions. Understanding the pathogenesis of CKD-induced muscle loss could lead to therapeutic interventions that prevent muscle wasting in CKD and potentially, other catabolic conditions.
Major findings: Insulin or IGF-1 resistance caused by CKD, acidosis, inflammation, glucocorticoids or cancer causes defects in insulin-stimulated intracellular signaling that suppresses IRS-1 activity leading to decreased phosphorylation of Akt (p-Akt). A low p-Akt activates caspase-3 which provides muscle proteins substrates of the ubiquitin-proteasome system (UPS). A low p-Akt also leads to decreased phosphorylation of forkhead transcription factors which enter the nucleus to stimulate the expression of atrogin-1/MAFbx and MuRF1, E3 ubiquitin ligases that can be associated with proteolysis of muscle cells by the UPS. Caspase-3 also stimulates proteasome-dependent proteolysis in muscle.
Summary: In CKD, diabetes, inflammatory conditions or in response to acidosis or excess glucocorticoids, insulin resistance develops, initiating reduced IRS-1/PI3K/Akt signaling. In CKD, this reduces p-Akt which stimulates muscle proteolysis by activating caspase-3 and the UPS. Second, caspase-3 cleaves actomyosin yielding substrates for the UPS and increased proteasome-mediated proteolysis. Third, p-Akt down-regulation suppresses myogenesis in CKD. Fourth, exercise in CKD stimulates insulin/IGF-1 signaling to reduce muscle atrophy. Lastly, there is evidence that microRNAs influence insulin signaling providing a potential opportunity to design therapeutic interventions. This article is part of a Directed Issue entitled: Molecular basis of muscle wasting.
Keywords: Acidosis; Cancer; Diabetes; Inflammation and glucocorticoid excess; Kidney failure.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
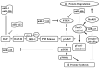
 microRNA
microRNA  Factors that stimulate muscle growth
Factors that stimulate muscle growth  Factors that inhibit muscle growth
Factors that inhibit muscle growth  Decrease or inhibit
Decrease or inhibit  Increase or stimulate
Increase or stimulate  Indirectly decrease or inhibit
Indirectly decrease or inhibitReferences
-
- Auclair D, Garrel DR, Chaouki Zerouala A, Ferland LH. Activation of the ubiquitin pathway in rat skeletal muscle by catabolic doses of glucocorticoids. Am J Physiol. 1997;272:C1007–1016. - PubMed
-
- Bailey JL, England BK, Long RC, Jr, Weissman J, Mitch WE. Experimentalacidemia and muscle cell pH in chronic acidosis and renal failure. Am J Physiol. 1995;269:C706–712. - PubMed
-
- Bailey JL, Zheng B, Hu Z, Price SR, Mitch WE. Chronic Kidney Disease Causes Defects in Signaling through the Insulin Receptor Substrate/Phosphatidylinositol 3-Kinase/Akt Pathway: Implications for Muscle Atrophy. J Am Soc Nephrol. 2006;17:1388–1394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

