Macrophage solubilization and cytotoxicity of indium-containing particles in vitro
- PMID: 23872580
- PMCID: PMC3807620
- DOI: 10.1093/toxsci/kft154
Macrophage solubilization and cytotoxicity of indium-containing particles in vitro
Abstract
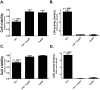
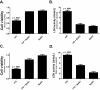
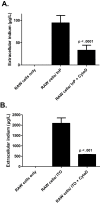
Indium-containing particles (ICPs) are used extensively in the microelectronics industry. Pulmonary toxicity is observed after inhalation exposure to ICPs; however, the mechanism(s) of pathogenesis is unclear. ICPs are insoluble at physiological pH and are initially engulfed by alveolar macrophages (and likely airway epithelial cells). We hypothesized that uptake of ICPs by macrophages followed by phagolysosomal acidification results in the solubilization of ICPs into cytotoxic indium ions. To address this, we characterized the in vitro cytotoxicity of indium phosphide (InP) or indium tin oxide (ITO) particles with macrophages (RAW cells) and lung-derived epithelial (LA-4) cells at 24h using metabolic (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) and membrane integrity (lactate dehydrogenase) assays. InP and ITO were readily phagocytosed by RAW and LA-4 cells; however, the particles were much more cytotoxic to RAW cells and cytotoxicity was dose dependent. Treatment of RAW cells with cytochalasin D (CytoD) blocked particle phagocytosis and reduced cytotoxicity. Treatment of RAW cells with bafilomycin A1, a specific inhibitor of phagolysosomal acidification, also reduced cytotoxicity but did not block particle uptake. Based on direct indium measurements, the concentration of ionic indium was increased in culture medium from RAW but not LA-4 cells following 24-h treatment with particles. Ionic indium derived from RAW cells was significantly reduced by treatment with CytoD. These data implicate macrophage uptake and solubilization of InP and ITO via phagolysosomal acidification as requisite for particle-induced cytotoxicity and the release of indium ions. This may apply to other ICPs and strongly supports the notion that ICPs require solubilization in order to be toxic.
Keywords: ITO; InP; indium; macrophage cytotoxicity.; solubilization.
Figures




References
-
- ACGIH (2007). Threshold Limit Values for Chemical Substances and Physical Agents, and Biological Exposure Indices, p. 35 Cincinnati, OH: American Conference of Governmental Industrial Hygienists;
-
- Chibli H., Carlini L., Park S., Dimitrijevic N. M., Nadeau J. L. (2011). Cytotoxicity of InP/ZnS quantum dots related to reactive oxygen species generation. Nanoscale 3, 2552–2559 - PubMed
-
- Chonan T., Taguchi O., Omae K. (2007). Interstitial pulmonary disorders in indium-processing workers. Eur. Respir. J 29, 317–324 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

