TARP γ-7 selectively enhances synaptic expression of calcium-permeable AMPARs
- PMID: 23872597
- PMCID: PMC3858651
- DOI: 10.1038/nn.3473
TARP γ-7 selectively enhances synaptic expression of calcium-permeable AMPARs
Abstract
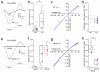
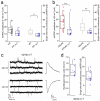
Regulation of calcium-permeable AMPA receptors (CP-AMPARs) is crucial in normal synaptic function and neurological disease states. Although transmembrane AMPAR regulatory proteins (TARPs) such as stargazin (γ-2) modulate the properties of calcium-impermeable AMPARs (CI-AMPARs) and promote their synaptic targeting, the TARP-specific rules governing CP-AMPAR synaptic trafficking remain unclear. We used RNA interference to manipulate AMPAR-subunit and TARP expression in γ-2-lacking stargazer cerebellar granule cells--the classic model of TARP deficiency. We found that TARP γ-7 selectively enhanced the synaptic expression of CP-AMPARs and suppressed CI-AMPARs, identifying a pivotal role of γ-7 in regulating the prevalence of CP-AMPARs. In the absence of associated TARPs, both CP-AMPARs and CI-AMPARs were able to localize to synapses and mediate transmission, although their properties were altered. Our results also establish that TARPed synaptic receptors in granule cells require both γ-2 and γ-7 and reveal an unexpected basis for the loss of AMPAR-mediated transmission in stargazer mice.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

