Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor
- PMID: 23873040
- PMCID: PMC3758448
- DOI: 10.1038/nature12358
Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor
Abstract
Co-development of the cardiovascular and pulmonary systems is a recent evolutionary adaption to terrestrial life that couples cardiac output with the gas exchange function of the lung. Here we show that the murine pulmonary vasculature develops even in the absence of lung development. We have identified a population of multipotent cardiopulmonary mesoderm progenitors (CPPs) within the posterior pole of the heart that are marked by the expression of Wnt2, Gli1 and Isl1. We show that CPPs arise from cardiac progenitors before lung development. Lineage tracing and clonal analysis demonstrates that CPPs generate the mesoderm lineages within the cardiac inflow tract and lung including cardiomyocytes, pulmonary vascular and airway smooth muscle, proximal vascular endothelium, and pericyte-like cells. CPPs are regulated by hedgehog expression from the foregut endoderm, which is required for connection of the pulmonary vasculature to the heart. Together, these studies identify a novel population of multipotent cardiopulmonary progenitors that coordinates heart and lung co-development that is required for adaptation to terrestrial existence.
Conflict of interest statement
The authors declare no competing financial interests
Figures
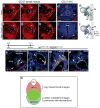
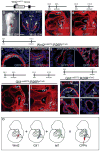

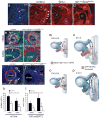
References
-
- Moses KA, DeMayo F, Braun RM, Reecy JL, Schwartz RJ. Embryonic expression of an Nkx2-5/Cre gene using ROSA26 reporter mice. Genesis. 2001;31:176–180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL087825/HL/NHLBI NIH HHS/United States
- P30 NS047101/NS/NINDS NIH HHS/United States
- T32 HL007586/HL/NHLBI NIH HHS/United States
- U01 HL107442/HL/NHLBI NIH HHS/United States
- R01 HL074066/HL/NHLBI NIH HHS/United States
- HL110942/HL/NHLBI NIH HHS/United States
- U01 HL110942/HL/NHLBI NIH HHS/United States
- T32 HL007843/HL/NHLBI NIH HHS/United States
- HL100405/HL/NHLBI NIH HHS/United States
- DP1 HL117649/HL/NHLBI NIH HHS/United States
- HL117649/HL/NHLBI NIH HHS/United States
- U01 HL100405/HL/NHLBI NIH HHS/United States
- HL087825/HL/NHLBI NIH HHS/United States
- T32 HL07586-23/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

