Genetic influences on plasma CFH and CFHR1 concentrations and their role in susceptibility to age-related macular degeneration
- PMID: 23873044
- PMCID: PMC3820139
- DOI: 10.1093/hmg/ddt336
Genetic influences on plasma CFH and CFHR1 concentrations and their role in susceptibility to age-related macular degeneration
Abstract
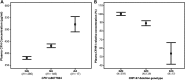
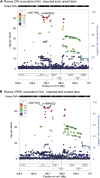
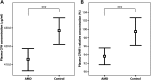
It is a longstanding puzzle why non-coding variants in the complement factor H (CFH) gene are more strongly associated with age-related macular degeneration (AMD) than functional coding variants that directly influence the alternative complement pathway. The situation is complicated by tight genetic associations across the region, including the adjacent CFH-related genes CFHR3 and CFHR1, which may themselves influence the alternative complement pathway and are contained within a common deletion (CNP147) which is associated with protection against AMD. It is unclear whether this association is mediated through a protective effect of low plasma CFHR1 concentrations, high plasma CFH or both. We examined the triangular relationships of CFH/CFHR3/CFHR1 genotype, plasma CFH or CFHR1 concentrations and AMD susceptibility in combined case-control (1256 cases, 1020 controls) and cross-sectional population (n = 1004) studies and carried out genome-wide association studies of plasma CFH and CFHR1 concentrations. A non-coding CFH SNP (rs6677604) and the CNP147 deletion were strongly correlated both with each other and with plasma CFH and CFHR1 concentrations. The plasma CFH-raising rs6677604 allele and raised plasma CFH concentration were each associated with AMD protection. In contrast, the protective association of the CNP147 deletion with AMD was not mediated by low plasma CFHR1, since AMD-free controls showed increased plasma CFHR1 compared with cases, but it may be mediated by the association of CNP147 with raised plasma CFH concentration. The results are most consistent with a regulatory locus within a 32 kb region of the CFH gene, with a major effect on plasma CFH concentration and AMD susceptibility.
Figures


References
-
- Foster A., Resnikoff S. The impact of Vision 2020 on global blindness. Eye. 2005;19:1133–1135. doi:10.1038/sj.eye.6701973. - DOI - PubMed
-
- Penfold P., Provis JM. Macular Degeneration. Berlin: Springer; 2005.
-
- Pieramici D.J., Rabena M.D. Anti-VEGF therapy: comparison of current and future agents. Eye. 2008;22:1330–1336. doi:10.1038/eye.2008.88. - DOI - PubMed
-
- Despriet D.D., Klaver C.C., Witteman J.C., Bergen A.A., Kardys I., de Maat M.P., Boekhoorn S.S., Vingerling J.R., Hofman A., Oostra B.A., et al. Complement factor H polymorphism, complement activators, and risk of age-related macular degeneration. JAMA. 2006;296:301–309. doi:10.1001/jama.296.3.301. - DOI - PubMed
-
- Seddon J.M., Francis P.J., George S., Schultz D.W., Rosner B., Klein M.L. Association of CFH Y402H and LOC387715 A69S with progression of age-related macular degeneration. JAMA. 2007;297:1793–1800. doi:10.1001/jama.297.16.1793. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

