Synergy between chemotherapeutic agents and CTLA-4 blockade in preclinical tumor models
- PMID: 23873089
- PMCID: PMC3755230
- DOI: 10.1007/s00262-013-1451-5
Synergy between chemotherapeutic agents and CTLA-4 blockade in preclinical tumor models
Abstract
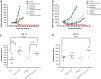
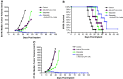
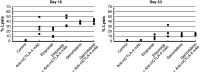
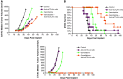
Ipilimumab, a cytotoxic T-lymphocyte antigen-4 (CTLA-4) binding agent, has proven to be an effective monotherapy for metastatic melanoma and has shown antitumor activity in trials when administered with other therapeutic agents. We hypothesized that the combination of ipilimumab with chemotherapeutic agents, such as ixabepilone, paclitaxel, etoposide, and gemcitabine, may produce therapeutic synergy based on distinct but complementary mechanisms of action for each drug and unique cellular targets. This concept was investigated using a mouse homolog of ipilimumab in preclinical murine tumor models, including SA1N fibrosarcoma, EMT-6 mammary carcinoma, M109 lung carcinoma, and CT-26 colon carcinoma. Results of CTLA-4 blockade in combination with one of various chemotherapeutic agents demonstrate that synergy occurs in settings where either agent alone was not effective in inducing tumor regression. Furthermore, when combined with CTLA-4 blockade, ixabepilone, etoposide, and gemcitabine elicited prolonged antitumor effects in some murine models with induction of a memory immune response. Future investigations are warranted to determine which specific chemo-immunotherapy combinations, if any, will produce synergistic antitumor effects in the clinical setting.
Conflict of interest statement
Maria Jure-Kunkel, Gregg Masters, Francis Lee, and John T. Hunt are current employees of BMS and have stock ownership in BMS; Emel Girit is a former employee of BMS and has stock ownership in BMS; Gennaro Dito is a current employee of BMS; Rachel Humphrey is a former employee of BMS, a consultant for Merck Serono and Johnson & Johnson, and a current employee of MethylGene, in which she has stock ownership.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

