Small molecules enable neurogenin 2 to efficiently convert human fibroblasts into cholinergic neurons
- PMID: 23873306
- PMCID: PMC3843951
- DOI: 10.1038/ncomms3183
Small molecules enable neurogenin 2 to efficiently convert human fibroblasts into cholinergic neurons
Abstract
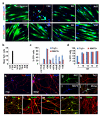
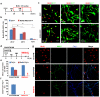
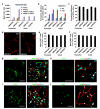
Cell fate can be reprogrammed by modifying intrinsic and extrinsic cues. Here we show that two small molecules (forskolin and dorsomorphin) enable the transcription factor Neurogenin 2 (NGN2) to convert human fetal lung fibroblasts into cholinergic neurons with high purity (>90%) and efficiency (up to 99% of NGN2-expressing cells). The conversion is direct without passing through a proliferative progenitor state. These human induced cholinergic neurons (hiCN) show mature electrophysiological properties and exhibit motor neuron-like features, including morphology, gene expression and the formation of functional neuromuscular junctions. Inclusion of an additional transcription factor, SOX11, also efficiently converts postnatal and adult skin fibroblasts from healthy and diseased human patients to cholinergic neurons. Taken together, this study identifies a simple and highly efficient strategy for reprogramming human fibroblasts to subtype-specific neurons. These findings offer a unique venue for investigating the molecular mechanisms underlying cellular plasticity and human neurodegenerative diseases.
Figures



Similar articles
-
Small Molecules Modulate Chromatin Accessibility to Promote NEUROG2-Mediated Fibroblast-to-Neuron Reprogramming.Stem Cell Reports. 2016 Nov 8;7(5):955-969. doi: 10.1016/j.stemcr.2016.09.013. Epub 2016 Oct 27. Stem Cell Reports. 2016. PMID: 28157484 Free PMC article.
-
Overexpression of basic helix-loop-helix transcription factors enhances neuronal differentiation of fetal human neural progenitor cells in various ways.Stem Cells Dev. 2012 Mar 1;21(4):539-53. doi: 10.1089/scd.2011.0079. Epub 2011 Jul 18. Stem Cells Dev. 2012. PMID: 21561385 Free PMC article.
-
Ngn2-Induced Differentiation of the NG108-15 Cell Line Enhances Motor Neuronal Differentiation and Neuromuscular Junction Formation.Biomolecules. 2025 Apr 29;15(5):637. doi: 10.3390/biom15050637. Biomolecules. 2025. PMID: 40427530 Free PMC article.
-
The zinc finger E-box-binding homeobox 1 (Zeb1) promotes the conversion of mouse fibroblasts into functional neurons.J Biol Chem. 2017 Aug 4;292(31):12959-12970. doi: 10.1074/jbc.M116.771493. Epub 2017 May 12. J Biol Chem. 2017. PMID: 28500132 Free PMC article.
-
Reprogramming the fate of human glioma cells to impede brain tumor development.Cell Death Dis. 2014 Oct 16;5(10):e1463. doi: 10.1038/cddis.2014.425. Cell Death Dis. 2014. PMID: 25321470 Free PMC article.
Cited by
-
MiR-124 and Small Molecules Synergistically Regulate the Generation of Neuronal Cells from Rat Cortical Reactive Astrocytes.Mol Neurobiol. 2021 May;58(5):2447-2464. doi: 10.1007/s12035-021-02345-6. Epub 2021 Mar 16. Mol Neurobiol. 2021. PMID: 33725319
-
Overcoming the hurdles for a reproducible generation of human functionally mature reprogrammed neurons.Exp Biol Med (Maywood). 2015 Jun;240(6):787-94. doi: 10.1177/1535370215577585. Epub 2015 Mar 18. Exp Biol Med (Maywood). 2015. PMID: 25790823 Free PMC article. Review.
-
Direct reprogramming of fibroblasts into renal tubular epithelial cells by defined transcription factors.Nat Cell Biol. 2016 Dec;18(12):1269-1280. doi: 10.1038/ncb3437. Epub 2016 Nov 7. Nat Cell Biol. 2016. PMID: 27820600
-
Direct Conversion of Human Urine Cells to Neurons by Small Molecules.Sci Rep. 2019 Nov 13;9(1):16707. doi: 10.1038/s41598-019-53007-6. Sci Rep. 2019. PMID: 31723223 Free PMC article.
-
Mechanistic Insights Into MicroRNA-Induced Neuronal Reprogramming of Human Adult Fibroblasts.Front Neurosci. 2018 Aug 2;12:522. doi: 10.3389/fnins.2018.00522. eCollection 2018. Front Neurosci. 2018. PMID: 30116172 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

