Lithothamnion muelleri controls inflammatory responses, target organ injury and lethality associated with graft-versus-host disease in mice
- PMID: 23873335
- PMCID: PMC3736440
- DOI: 10.3390/md11072595
Lithothamnion muelleri controls inflammatory responses, target organ injury and lethality associated with graft-versus-host disease in mice
Abstract
Lithothamnion muelleri (Hapalidiaceae) is a marine red alga, which is a member of a group of algae with anti-inflammatory, antitumor, and immunomodulatory properties. The present study evaluated the effects of treatment with Lithothamnion muelleri extract (LM) in a model of acute graft-versus-host disease (GVHD), using a model of adoptive splenocyte transfer from C57BL/6 donors into B6D2F1 recipient mice. Mice treated with LM showed reduced clinical signs of disease and mortality when compared with untreated mice. LM-treated mice had reduced tissue injury, less bacterial translocation, and decreased levels of proinflammatory cytokines and chemokines (interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), chemokine (C-C motif) ligand 2 (CCL2), chemokine (C-C motif) ligand 3 (CCL3) and chemokine (C-C motif) ligand 5 (CCL5)). The polysaccharide-rich fraction derived from LM could inhibit leukocyte rolling and adhesion in intestinal venules, as assessed by intravital microscopy. LM treatment did not impair the beneficial effects of graft-versus-leukaemia (GVL). Altogether, our studies suggest that treatment with Lithothamnion muelleri has a potential therapeutic application in GVHD treatment.
Figures
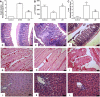


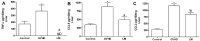


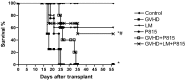
References
-
- Pasquini M.C., Wang Z., Horowitz M.M., Gale R.P. 2010 report from the Center for International Blood and Marrow Transplant Research (CIBMTR): Current uses and outcomes of hematopoietic cell transplants for blood and bone marrow disorders. Clin. Transpl. 2010;2010:87–105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

