Increased tissue transglutaminase activity contributes to central vascular stiffness in eNOS knockout mice
- PMID: 23873798
- PMCID: PMC3761342
- DOI: 10.1152/ajpheart.00103.2013
Increased tissue transglutaminase activity contributes to central vascular stiffness in eNOS knockout mice
Abstract
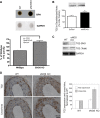
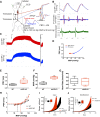
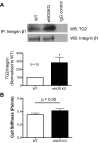
Nitric oxide (NO) can modulate arterial stiffness by regulating both functional and structural changes in the arterial wall. Tissue transglutaminase (TG2) has been shown to contribute to increased central aortic stiffness by catalyzing the cross-linking of matrix proteins. NO S-nitrosylates and constrains TG2 to the cytosolic compartment and thereby holds its cross-linking function latent. In the present study, the role of endothelial NO synthase (eNOS)-derived NO in regulating TG2 function was studied using eNOS knockout mice. Matrix-associated TG2 and TG2 cross-linking function were higher, whereas TG2 S-nitrosylation was lower in the eNOS(-/-) compared with wild-type (WT) mice. Pulse-wave velocity (PWV) and blood pressure measured noninvasively were elevated in the eNOS(-/-) compared with WT mice. Intact aortas and decellularized aortic tissue scaffolds of eNOS(-/-) mice were significantly stiffer, as determined by tensile testing. The carotid arteries of the eNOS(-/-) mice were also stiffer, as determined by pressure-dimension analysis. Invasive methods to determine the PWV-mean arterial pressure relationship showed that PWV in eNOS(-/-) and WT diverge at higher mean arterial pressure. Thus eNOS-derived NO regulates TG2 localization and function and contributes to vascular stiffness.
Keywords: S-nitrosylation; endothelial nitric oxide synthase; nitric oxide; pulse-wave velocity; tensile testing; tissue transglutaminase; vascular stiffness.
Figures

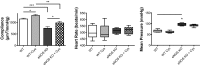
References
-
- Alcock J, Warren AY, Goodson YJ, Hill SJ, Khan RN, Lymn JS. Inhibition of tissue transglutaminase 2 attenuates contractility of pregnant human myometrium. Biol Reprod 84: 646–653, 2011 - PubMed
-
- Bakker EN, Buus CL, Spaan JA, Perree J, Ganga A, Rolf TM, Sorop O, Bramsen LH, Mulvany MJ, Vanbavel E. Small artery remodeling depends on tissue-type transglutaminase. Circ Res 96: 119–126, 2005 - PubMed
-
- Bakker EN, Pistea A, Spaan JA, Rolf T, de Vries CJ, van Rooijen N, Candi E, VanBavel E. Flow-dependent remodeling of small arteries in mice deficient for tissue-type transglutaminase: possible compensation by macrophage-derived factor XIII. Circ Res 99: 86–92, 2006 - PubMed
-
- Butlin M, Hammond A, Lindesay G, Viegas K, Avolio AP. In-vitro and in-vivo use of vasoactive agents in characterising aortic stiffness in rats: testing the assumptions. J Hypertension. In press
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

