Reduced microtubule acetylation in cystic fibrosis epithelial cells
- PMID: 23873844
- PMCID: PMC3763039
- DOI: 10.1152/ajplung.00411.2012
Reduced microtubule acetylation in cystic fibrosis epithelial cells
Abstract
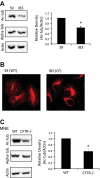
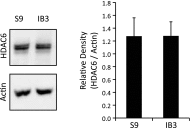
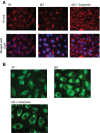
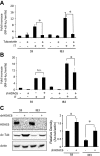
Dysfunctional cystic fibrosis transmembrane conductance regulator (CFTR) leads to many cellular consequences, including perinuclear accumulation of free cholesterol due to impaired endosomal transport. The hypothesis being tested is that CF-related perinuclear cholesterol accumulation due to disrupted endocytic trafficking occurs as a result of reduced microtubule (MT) acetylation. Here, it is identified that acetylated-α-tubulin (Ac-tub) content is reduced by ∼40% compared with respective wild-type controls in both cultured CF cell models (IB3) and primary Cftr-/- mouse nasal epithelial tissue. Histone deacetylase 6 (HDAC6) has been shown to regulate MT acetylation, which provides reasonable grounds to test its impact on reduced Ac-tub content on CF cellular phenotypes. Inhibition of HDAC6, either through tubastatin treatment or HDAC6 knockdown in CF cells, increases Ac-tub content and results in redistributed free cholesterol and reduced stimulation of NF-κB activity. Mechanistically, endoplasmic reticulum stress, which is widely reported in CF and leads to aggresome formation, is identified as a regulator of MT acetylation. F508del CFTR correction with C18 in primary airway epithelial cells restores MT acetylation and cholesterol transport. A significant role for phosphatidyl inositol-3 kinase p110α is also identified as a regulator of MT acetylation.
Keywords: cholesterol; cystic fibrosis; histone deacetylase 6; microtubule; phosphatidyl inositol-3 kinase p110α.
Figures


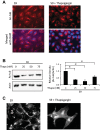
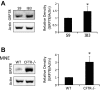
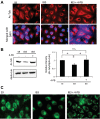
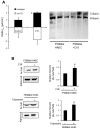

Similar articles
-
Electrochemical measurement of membrane cholesterol correlates with CFTR function and is HDAC6-dependent.J Cyst Fibros. 2019 Mar;18(2):175-181. doi: 10.1016/j.jcf.2018.06.005. Epub 2018 Jun 22. J Cyst Fibros. 2019. PMID: 29941319 Free PMC article.
-
MeCP2 deficiency is associated with reduced levels of tubulin acetylation and can be restored using HDAC6 inhibitors.J Mol Med (Berl). 2015 Jan;93(1):63-72. doi: 10.1007/s00109-014-1202-x. Epub 2014 Sep 12. J Mol Med (Berl). 2015. PMID: 25209898
-
Inhibition of HDAC6 deacetylase activity increases its binding with microtubules and suppresses microtubule dynamic instability in MCF-7 cells.J Biol Chem. 2013 Aug 2;288(31):22516-26. doi: 10.1074/jbc.M113.489328. Epub 2013 Jun 24. J Biol Chem. 2013. PMID: 23798680 Free PMC article.
-
Role of Exchange Protein Activated by cAMP 1 in Regulating Rates of Microtubule Formation in Cystic Fibrosis Epithelial Cells.Am J Respir Cell Mol Biol. 2015 Dec;53(6):853-62. doi: 10.1165/rcmb.2014-0462OC. Am J Respir Cell Mol Biol. 2015. PMID: 25955407 Free PMC article.
-
Chasing a Breath of Fresh Air in Cystic Fibrosis (CF): Therapeutic Potential of Selective HDAC6 Inhibitors to Tackle Multiple Pathways in CF Pathophysiology.J Med Chem. 2022 Feb 24;65(4):3080-3097. doi: 10.1021/acs.jmedchem.1c02067. Epub 2022 Feb 11. J Med Chem. 2022. PMID: 35148101 Free PMC article. Review.
Cited by
-
Gene expression in transformed lymphocytes reveals variation in endomembrane and HLA pathways modifying cystic fibrosis pulmonary phenotypes.Am J Hum Genet. 2015 Feb 5;96(2):318-28. doi: 10.1016/j.ajhg.2014.12.022. Epub 2015 Jan 29. Am J Hum Genet. 2015. PMID: 25640674 Free PMC article.
-
Point-of-care ultrasound-guided submucosal paclitaxel injection in tracheal stenosis model.J Transl Int Med. 2023 May 7;11(1):70-80. doi: 10.2478/jtim-2022-0044. eCollection 2023 Mar. J Transl Int Med. 2023. PMID: 37533845 Free PMC article.
-
Cellular chloride and bicarbonate retention alters intracellular pH regulation in Cftr KO crypt epithelium.Am J Physiol Gastrointest Liver Physiol. 2016 Jan 15;310(2):G70-80. doi: 10.1152/ajpgi.00236.2015. Epub 2015 Nov 5. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 26542396 Free PMC article.
-
Research advances in molecular mechanisms underlying the pathogenesis of cystic fibrosis: From technical improvement to clinical applications (Review).Mol Med Rep. 2020 Dec;22(6):4992-5002. doi: 10.3892/mmr.2020.11607. Epub 2020 Oct 16. Mol Med Rep. 2020. PMID: 33173976 Free PMC article. Review.
-
Histone deacetylase inhibitors (HDACi) increase expression of KCa2.3 (SK3) in primary microvascular endothelial cells.Am J Physiol Cell Physiol. 2022 Mar 1;322(3):C338-C353. doi: 10.1152/ajpcell.00409.2021. Epub 2022 Jan 19. Am J Physiol Cell Physiol. 2022. PMID: 35044858 Free PMC article.
References
-
- Ammerpohl O, Trauzold A, Schniewind B, Griep U, Pilarsky C, Grutzmann R, Saeger HD, Janssen O, Sipos B, Kloppel G, Kalthoff H. Complementary effects of HDAC inhibitor 4-PB on gap junction communication and cellular export mechanisms support restoration of chemosensitivity of PDAC cells. Br J Cancer 96: 73–81, 2007 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

