Efficacy of as-needed nalmefene in alcohol-dependent patients with at least a high drinking risk level: results from a subgroup analysis of two randomized controlled 6-month studies
- PMID: 23873853
- PMCID: PMC3746807
- DOI: 10.1093/alcalc/agt061
Efficacy of as-needed nalmefene in alcohol-dependent patients with at least a high drinking risk level: results from a subgroup analysis of two randomized controlled 6-month studies
Erratum in
- Alcohol Alcohol. 2013 Nov-Dec;48(6):746
Abstract
Aims: The aim of the study was to investigate the efficacy and safety of as-needed use of nalmefene 18 mg versus placebo in reducing alcohol consumption in patients who did not reduce their alcohol consumption after an initial assessment, i.e. the pooled subgroup of patients with at least a high drinking risk level (men: >60 g/day; women: >40 g/day) at both screening and randomization from the two randomized controlled 6-month studies ESENSE 1 (NCT00811720) and ESENSE 2 (NCT00812461).
Methods: Nalmefene 18 mg and placebo were taken on an as-needed basis. All the patients also received a motivational and adherence-enhancing intervention (BRENDA). The co-primary outcomes were number of heavy drinking days (HDDs) and mean total alcohol consumption (g/day) in Month 6 measured using the Timeline Follow-back method. Additionally, data on clinical improvement, liver function and safety were collected throughout the study.
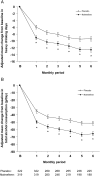
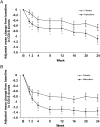
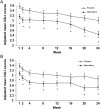
Results: The pooled population consisted of 667 patients: placebo n = 332; nalmefene n = 335. There was a superior effect of nalmefene compared with placebo in reducing the number of HDDs [treatment difference: -3.2 days (95% CI: -4.8; -1.6); P < 0.0001] and total alcohol consumption [treatment difference: -14.3 g/day (-20.8; -7.8); P < 0.0001] at Month 6. Improvements in clinical status and liver parameters were greater in the nalmefene group compared with the placebo group. Adverse events and adverse events leading to dropout were more common with nalmefene than placebo.
Conclusion: As-needed nalmefene was efficacious in reducing alcohol consumption in patients with at least a high drinking risk level at both screening and randomization, and the effect in this subgroup was larger than in the total population.
Figures

References
-
- Alonso J, Angermeyer MC, Bernert S, et al. Use of mental health services in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand Suppl. 2004;420:47–54. - PubMed
-
- American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders. 4th edn. Washington, DC: American Psychiatric Association; 2000. Text Revision (DSM-IV-TR)
-
- Bart G, Schluger JH, Borg L, et al. Nalmefene induced elevation in serum prolactin in normal human volunteers: partial kappa opioid agonist activity? Neuropsychopharmacology. 2005;30:2254–62. doi:10.1038/sj.npp.1300811. - DOI - PubMed
-
- Beich A, Thorsen T, Rollnick S. Screening in brief intervention trials targeting excessive drinkers in general practice: systematic review and meta-analysis. BMJ. 2003;327:536–42. doi:10.1136/bmj.327.7414.536. - DOI - PMC - PubMed
-
- Cohen E, Feinn R, Arias A, et al. Alcohol treatment utilization: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2007;86:214–21. doi:10.1016/j.drugalcdep.2006.06.008. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

