Physiological consequences of multiple-target regulation by the small RNA SgrS in Escherichia coli
- PMID: 23873911
- PMCID: PMC3807494
- DOI: 10.1128/JB.00722-13
Physiological consequences of multiple-target regulation by the small RNA SgrS in Escherichia coli
Abstract
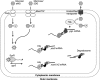
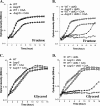
Cells use complex mechanisms to regulate glucose transport and metabolism to achieve optimal energy and biomass production while avoiding accumulation of toxic metabolites. Glucose transport and glycolytic metabolism carry the risk of the buildup of phosphosugars, which can inhibit growth at high concentrations. Many enteric bacteria cope with phosphosugar accumulation and associated stress (i.e., sugar-phosphate stress) by producing a small RNA (sRNA) regulator, SgrS, which decreases phosphosugar accumulation in part by repressing translation of sugar transporter mRNAs (ptsG and manXYZ) and enhancing translation of a sugar phosphatase mRNA (yigL). Despite a molecular understanding of individual target regulation by SgrS, previously little was known about how coordinated regulation of these multiple targets contributes to the rescue of cell growth during sugar-phosphate stress. This study examines how SgrS regulation of different targets impacts growth under different nutritional conditions when sugar-phosphate stress is induced. The severity of stress-associated growth inhibition depended on nutrient availability. Stress in nutrient-rich media necessitated SgrS regulation of only sugar transporter mRNAs (ptsG or manXYZ). However, repression of transporter mRNAs was insufficient for growth rescue during stress in nutrient-poor media; here SgrS regulation of the phosphatase (yigL) and as-yet-undefined targets also contributed to growth rescue. The results of this study imply that regulation of only a subset of an sRNA's targets may be important in a given environment. Further, the results suggest that SgrS and perhaps other sRNAs are flexible regulators that modulate expression of multigene regulons to allow cells to adapt to an array of stress conditions.
Figures



Similar articles
-
The small RNA SgrS controls sugar-phosphate accumulation by regulating multiple PTS genes.Nucleic Acids Res. 2011 May;39(9):3806-19. doi: 10.1093/nar/gkq1219. Epub 2011 Jan 17. Nucleic Acids Res. 2011. PMID: 21245045 Free PMC article.
-
A minimal base-pairing region of a bacterial small RNA SgrS required for translational repression of ptsG mRNA.Mol Microbiol. 2010 May;76(3):782-92. doi: 10.1111/j.1365-2958.2010.07141.x. Epub 2010 Mar 25. Mol Microbiol. 2010. PMID: 20345651
-
Regulation and function of Escherichia coli sugar efflux transporter A (SetA) during glucose-phosphate stress.J Bacteriol. 2011 Jan;193(1):143-53. doi: 10.1128/JB.01008-10. Epub 2010 Oct 22. J Bacteriol. 2011. PMID: 20971900 Free PMC article.
-
The small RNA SgrS: roles in metabolism and pathogenesis of enteric bacteria.Front Cell Infect Microbiol. 2014 May 8;4:61. doi: 10.3389/fcimb.2014.00061. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 24847473 Free PMC article. Review.
-
Physiological consequences of small RNA-mediated regulation of glucose-phosphate stress.Curr Opin Microbiol. 2007 Apr;10(2):146-51. doi: 10.1016/j.mib.2007.03.011. Epub 2007 Mar 23. Curr Opin Microbiol. 2007. PMID: 17383224 Review.
Cited by
-
Systematic characterization of artificial small RNA-mediated inhibition of Escherichia coli growth.RNA Biol. 2017 Feb;14(2):206-218. doi: 10.1080/15476286.2016.1270001. Epub 2016 Dec 16. RNA Biol. 2017. PMID: 27981881 Free PMC article.
-
Stringent Response Regulators Contribute to Recovery from Glucose Phosphate Stress in Escherichia coli.Appl Environ Microbiol. 2017 Dec 1;83(24):e01636-17. doi: 10.1128/AEM.01636-17. Print 2017 Dec 15. Appl Environ Microbiol. 2017. PMID: 28986375 Free PMC article.
-
Cryptic-Prophage-Encoded Small Protein DicB Protects Escherichia coli from Phage Infection by Inhibiting Inner Membrane Receptor Proteins.J Bacteriol. 2019 Nov 5;201(23):e00475-19. doi: 10.1128/JB.00475-19. Print 2019 Dec 1. J Bacteriol. 2019. PMID: 31527115 Free PMC article.
-
Translational regulation by bacterial small RNAs via an unusual Hfq-dependent mechanism.Nucleic Acids Res. 2018 Mar 16;46(5):2585-2599. doi: 10.1093/nar/gkx1286. Nucleic Acids Res. 2018. PMID: 29294046 Free PMC article.
-
Deciphering the interplay between two independent functions of the small RNA regulator SgrS in Salmonella.J Bacteriol. 2013 Oct;195(20):4620-30. doi: 10.1128/JB.00586-13. Epub 2013 Aug 9. J Bacteriol. 2013. PMID: 23935052 Free PMC article.
References
-
- Plumbridge J. 1998. Expression of ptsG, the gene for the major glucose PTS transporter in Escherichia coli, is repressed by Mlc and induced by growth on glucose. Mol. Microbiol. 29:1053–1063 - PubMed
-
- Tanaka Y, Kimata K, Inada T, Tagami H, Aiba H. 1999. Negative regulation of the pts operon by Mlc: mechanism underlying glucose induction in Escherichia coli. Genes Cells 4:391–399 - PubMed
-
- Vanderpool CK, Gottesman S. 2004. Involvement of a novel transcriptional activator and small RNA in post-transcriptional regulation of the glucose phosphoenolpyruvate phosphotransferase system. Mol. Microbiol. 54:1076–1089 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

