Effects of low PBP2b levels on cell morphology and peptidoglycan composition in Streptococcus pneumoniae R6
- PMID: 23873916
- PMCID: PMC3807472
- DOI: 10.1128/JB.00184-13
Effects of low PBP2b levels on cell morphology and peptidoglycan composition in Streptococcus pneumoniae R6
Abstract
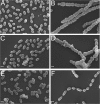
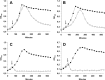
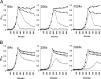
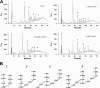
Streptococcus pneumoniae produces two class B penicillin-binding proteins, PBP2x and PBP2b, both of which are essential. It is generally assumed that PBP2x is specifically involved in septum formation, while PBP2b is dedicated to peripheral cell wall synthesis. However, little experimental evidence exists to substantiate this belief. In the present study, we obtained evidence that strongly supports the view that PBP2b is essential for peripheral peptidoglycan synthesis. Depletion of PBP2b expression gave rise to long chains of cells in which individual cells were compressed in the direction of the long axis and looked lentil shaped. This morphological change is consistent with a role for pneumococcal PBP2b in the synthesis of the lateral cell wall. Depletion of PBP2x, on the other hand, resulted in lemon-shaped and some elongated cells with a thickened midcell region. Low PBP2b levels gave rise to changes in the peptidoglycan layer that made pneumococci sensitive to exogenously added LytA during logarithmic growth and refractory to chain dispersion upon addition of LytB. Interestingly, analysis of the cell wall composition of PBP2b-depleted pneumococci revealed that they had a larger proportion of branched stem peptides in their peptidoglycan than the corresponding undepleted cells. Furthermore, MurM-deficient mutants, i.e., mutants lacking the ability to synthesize branched muropeptides, were found to require much higher levels of PBP2b to sustain growth than those required by MurM-proficient strains. These findings might help to explain why increased incorporation of branched muropeptides is required for high-level beta-lactam resistance in S. pneumoniae.
Figures


References
-
- Zapun A, Vernet T, Pinho MG. 2008. The different shapes of cocci. FEMS Microbiol. Rev. 32:345–360 - PubMed
-
- Sauvage E, Kerff F, Terrak M, Ayala JA, Charlier P. 2008. The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 32:234–258 - PubMed
-
- Kell CM, Sharma UK, Dowson CG, Town C, Balganesh TS, Spratt BG. 1993. Deletion analysis of the essentiality of penicillin-binding proteins 1A, 2B and 2X of Streptococcus pneumoniae. FEMS Microbiol. Lett. 106:171–175 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

