A randomized phase II clinical trial of high-dose nicotine patch therapy for smokeless tobacco users
- PMID: 23873976
- PMCID: PMC3819979
- DOI: 10.1093/ntr/ntt097
A randomized phase II clinical trial of high-dose nicotine patch therapy for smokeless tobacco users
Abstract
Introduction: Nicotine patch therapy has not been shown to be efficacious for increasing long-term (≥6 months) tobacco abstinence rates among smokeless tobacco (ST) users. Higher doses of nicotine patch therapy may be needed to increase tobacco abstinence rates in this population of tobacco users.
Methods: We randomized ST users who used ≥3 cans/pouches per week to either 8 weeks of high-dose nicotine patch therapy (42mg/day) or matching placebo patch. Subjects were followed for 6 months after randomization.
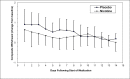
Results: Fifty-two subjects were randomized. Compared with placebo, high-dose nicotine patch therapy was associated with significantly higher prolonged tobacco abstinence at end-of-treatment (44% vs. 22%, odds ratio [OR] = 2.7, p = .050) and 3 months (40% vs. 19%, OR = 2.9, p = .047). High-dose nicotine patch therapy was associated with significant weight gain attenuation among tobacco abstinence subjects at 3 months (p = .013) and 6 months (p = .018). Compared with placebo, high-dose nicotine patch therapy was associated with nonsignificantly lower nicotine withdrawal scores. Adverse events were not significantly increased with high-dose nicotine patch therapy.
Conclusions: High-dose nicotine patch therapy is safe and increases short-term tobacco abstinence rates among ST users who use ≥3 cans/pouches per week. High-dose nicotine patch therapy is associated with significant long-term attenuation of weight gain. Future studies to investigate the long-term efficacy of high-dose nicotine patch therapy and the comparative efficacy of this approach compared with standard nicotine patch doses for ST users seems warranted.
Figures
References
-
- Benowitz N. L., Ahijevych K., Hall S., Hansson A., Henningfield J., Hurt R. D. … Velicer W. (2002). Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research, 4, 149–159 doi:10.1080/ 14622200210123581 - PubMed
-
- Benowitz N. L., Porchet H., Sheiner L., Jacob P., III (1988). Nicotine absorption and cardiovascular effects with smokeless tobacco use: Comparison with cigarettes and nicotine gum. Clinical Pharmacology and Therapeutics, 44, 23–28 doi:10.1038/clpt.1988.107 - PubMed
-
- Clark M. M., Decker P. A., Offord K. P., Patten C. A., Vickers K. S., Croghan I. T. … Dale L. C. (2004). Weight concerns among male smokers. Addictive Behaviors, 29, 1637–1641 doi:10.1016/j.addbeh.2004.02.034 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical