Adaptive responses to tissue injury: role of heme oxygenase-1
- PMID: 23874015
- PMCID: PMC3715920
Adaptive responses to tissue injury: role of heme oxygenase-1
Abstract
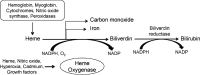
Tissue injury may result as a consequence of a physical, chemical, or biological insult. Such injury recruits an adaptive response to restore homeostasis and protect against further injury. One of the most prompt protective and adaptive responses by all tissues is the robust activation of the highly inducible, anti-inflammatory, anti-oxidant, and anti-apoptotic protein, heme oxygenase-1 (HO-1). HO-1, a microsomal enzyme, catalyzes the breakdown of pro-oxidant heme, which is released from heme proteins to equimolar quantities of iron, carbon monoxide, and biliverdin. Biliverdin is converted to bilirubin by biliverdin reductase. The beneficial effects of HO-1 expression are not merely due to heme degradation but are also attributed to the cytoprotective properties of the byproducts of the reaction. Manipulation of this enzymatic system in a myriad of disease models has provided substantial evidence to support its role as a cytoprotective enzyme and is therefore an emerging therapeutic molecule.
Conflict of interest statement
Potential Conflicts of Interest: None disclosed.
Figures
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
