Negative regulation of type I IFN expression by OASL1 permits chronic viral infection and CD8⁺ T-cell exhaustion
- PMID: 23874199
- PMCID: PMC3715418
- DOI: 10.1371/journal.ppat.1003478
Negative regulation of type I IFN expression by OASL1 permits chronic viral infection and CD8⁺ T-cell exhaustion
Abstract
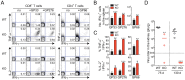
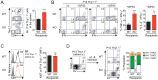
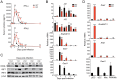
The type I interferons (IFN-Is) are critical not only in early viral control but also in prolonged T-cell immune responses. However, chronic viral infections such as those of human immunodeficiency virus (HIV) and hepatitis C virus (HCV) in humans and lymphocytic choriomeningitis virus (LCMV) in mice overcome this early IFN-I barrier and induce viral persistence and exhaustion of T-cell function. Although various T-cell-intrinsic and -extrinsic factors are known to contribute to induction of chronic conditions, the roles of IFN-I negative regulators in chronic viral infections have been largely unexplored. Herein, we explored whether 2'-5' oligoadenylate synthetase-like 1 (OASL1), a recently defined IFN-I negative regulator, plays a key role in the virus-specific T-cell response and viral defense against chronic LCMV. To this end, we infected Oasl1 knockout and wild-type mice with LCMV CL-13 (a chronic virus) and monitored T-cell responses, serum cytokine levels, and viral titers. LCMV CL-13-infected Oasl1 KO mice displayed a sustained level of serum IFN-I, which was primarily produced by splenic plasmacytoid dendritic cells, during the very early phase of infection (2-3 days post-infection). Oasl1 deficiency also led to the accelerated elimination of viremia and induction of a functional antiviral CD8 T-cell response, which critically depended on IFN-I receptor signaling. Together, these results demonstrate that OASL1-mediated negative regulation of IFN-I production at an early phase of infection permits viral persistence and suppresses T-cell function, suggesting that IFN-I negative regulators, including OASL1, could be exciting new targets for preventing chronic viral infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Lee MS, Kim YJ (2007) Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem 76: 447–480. - PubMed
-
- Barbalat R, Ewald SE, Mouchess ML, Barton GM (2011) Nucleic acid recognition by the innate immune system. Annu Rev Immunol 29: 185–214. - PubMed
-
- Kawai T, Akira S (2006) Innate immune recognition of viral infection. Nat Immunol 7: 131–137. - PubMed
-
- Iwasaki A, Medzhitov R (2004) Toll-like receptor control of the adaptive immune responses. Nat Immunol 5: 987–995. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

