Cytotoxic T cells mediate pathology and metastasis in cutaneous leishmaniasis
- PMID: 23874205
- PMCID: PMC3715507
- DOI: 10.1371/journal.ppat.1003504
Cytotoxic T cells mediate pathology and metastasis in cutaneous leishmaniasis
Abstract
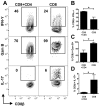
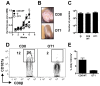
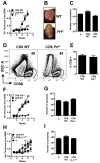
Disease progression in response to infection can be strongly influenced by both pathogen burden and infection-induced immunopathology. While current therapeutics focus on augmenting protective immune responses, identifying therapeutics that reduce infection-induced immunopathology are clearly warranted. Despite the apparent protective role for murine CD8⁺ T cells following infection with the intracellular parasite Leishmania, CD8⁺ T cells have been paradoxically linked to immunopathological responses in human cutaneous leishmaniasis. Transcriptome analysis of lesions from Leishmania braziliensis patients revealed that genes associated with the cytolytic pathway are highly expressed and CD8⁺ T cells from lesions exhibited a cytolytic phenotype. To determine if CD8⁺ T cells play a causal role in disease, we turned to a murine model. These studies revealed that disease progression and metastasis in L. braziliensis infected mice was independent of parasite burden and was instead directly associated with the presence of CD8⁺ T cells. In mice with severe pathology, we visualized CD8⁺ T cell degranulation and lysis of L. braziliensis infected cells. Finally, in contrast to wild-type CD8⁺ T cells, perforin-deficient cells failed to induce disease. Thus, we show for the first time that cytolytic CD8⁺ T cells mediate immunopathology and drive the development of metastatic lesions in cutaneous leishmaniasis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Ortega C, Fernandez AS, Carrillo JM, Romero P, Molina IJ, et al. (2009) IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. Journal of leukocyte biology 86: 435–443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

