FGF signalling regulates chromatin organisation during neural differentiation via mechanisms that can be uncoupled from transcription
- PMID: 23874217
- PMCID: PMC3715432
- DOI: 10.1371/journal.pgen.1003614
FGF signalling regulates chromatin organisation during neural differentiation via mechanisms that can be uncoupled from transcription
Erratum in
-
Correction: FGF Signalling Regulates Chromatin Organisation during Neural Differentiation via Mechanisms that Can Be Uncoupled from Transcription.PLoS Genet. 2013 Aug 12;9(8):10.1371/annotation/c066bb84-13ea-4b36-a481-f149df8ce929. doi: 10.1371/annotation/c066bb84-13ea-4b36-a481-f149df8ce929. eCollection 2013 Aug. PLoS Genet. 2013. PMID: 29364922 Free PMC article.
Abstract
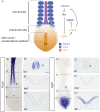
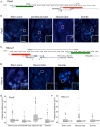
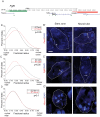
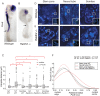
Changes in higher order chromatin organisation have been linked to transcriptional regulation; however, little is known about how such organisation alters during embryonic development or how it is regulated by extrinsic signals. Here we analyse changes in chromatin organisation as neural differentiation progresses, exploiting the clear spatial separation of the temporal events of differentiation along the elongating body axis of the mouse embryo. Combining fluorescence in situ hybridisation with super-resolution structured illumination microscopy, we show that chromatin around key differentiation gene loci Pax6 and Irx3 undergoes both decompaction and displacement towards the nuclear centre coincident with transcriptional onset. Conversely, down-regulation of Fgf8 as neural differentiation commences correlates with a more peripheral nuclear position of this locus. During normal neural differentiation, fibroblast growth factor (FGF) signalling is repressed by retinoic acid, and this vitamin A derivative is further required for transcription of neural genes. We show here that exposure to retinoic acid or inhibition of FGF signalling promotes precocious decompaction and central nuclear positioning of differentiation gene loci. Using the Raldh2 mutant as a model for retinoid deficiency, we further find that such changes in higher order chromatin organisation are dependent on retinoid signalling. In this retinoid deficient condition, FGF signalling persists ectopically in the elongating body, and importantly, we find that inhibiting FGF receptor (FGFR) signalling in Raldh2-/- embryos does not rescue differentiation gene transcription, but does elicit both chromatin decompaction and nuclear position change. These findings demonstrate that regulation of higher order chromatin organisation during differentiation in the embryo can be uncoupled from the machinery that promotes transcription and, for the first time, identify FGF as an extrinsic signal that can direct chromatin compaction and nuclear organisation of gene loci.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
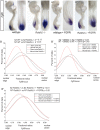
Similar articles
-
Retinoic acid orchestrates fibroblast growth factor signalling to drive embryonic stem cell differentiation.Development. 2010 Mar;137(6):881-90. doi: 10.1242/dev.043117. Development. 2010. PMID: 20179094 Free PMC article.
-
Loss- and gain-of-function analyses reveal targets of Pax6 in the developing mouse telencephalon.Mol Cell Neurosci. 2007 Jan;34(1):99-119. doi: 10.1016/j.mcn.2006.10.008. Epub 2006 Dec 8. Mol Cell Neurosci. 2007. PMID: 17158062
-
Multiple points of interaction between retinoic acid and FGF signaling during embryonic axis formation.Development. 2004 Jun;131(11):2653-67. doi: 10.1242/dev.01129. Epub 2004 May 5. Development. 2004. PMID: 15128657
-
Concise review: Pax6 transcription factor contributes to both embryonic and adult neurogenesis as a multifunctional regulator.Stem Cells. 2008 Jul;26(7):1663-72. doi: 10.1634/stemcells.2007-0884. Epub 2008 May 8. Stem Cells. 2008. PMID: 18467663 Review.
-
Mechanisms of retinoic acid signalling and its roles in organ and limb development.Nat Rev Mol Cell Biol. 2015 Feb;16(2):110-23. doi: 10.1038/nrm3932. Epub 2015 Jan 5. Nat Rev Mol Cell Biol. 2015. PMID: 25560970 Free PMC article. Review.
Cited by
-
Getting in touch with your senses: Mechanisms specifying sensory interneurons in the dorsal spinal cord.WIREs Mech Dis. 2021 Sep;13(5):e1520. doi: 10.1002/wsbm.1520. Epub 2021 Feb 25. WIREs Mech Dis. 2021. PMID: 34730293 Free PMC article. Review.
-
ChAT-positive neurons participate in subventricular zone neurogenesis after middle cerebral artery occlusion in mice.Behav Brain Res. 2017 Jan 1;316:145-151. doi: 10.1016/j.bbr.2016.09.007. Epub 2016 Sep 5. Behav Brain Res. 2017. PMID: 27609645 Free PMC article.
-
Hmgb2 improves astrocyte to neuron conversion by increasing the chromatin accessibility of genes associated with neuronal maturation in a proneuronal factor-dependent manner.Genome Biol. 2025 Apr 17;26(1):100. doi: 10.1186/s13059-025-03556-z. Genome Biol. 2025. PMID: 40247387 Free PMC article.
-
A tissue-specific self-interacting chromatin domain forms independently of enhancer-promoter interactions.Nat Commun. 2018 Sep 21;9(1):3849. doi: 10.1038/s41467-018-06248-4. Nat Commun. 2018. PMID: 30242161 Free PMC article.
-
Spatial genome organization: contrasting views from chromosome conformation capture and fluorescence in situ hybridization.Genes Dev. 2014 Dec 15;28(24):2778-91. doi: 10.1101/gad.251694.114. Genes Dev. 2014. PMID: 25512564 Free PMC article.
References
-
- Wilson V, Olivera-Martinez I, Storey KG (2009) Stem cells, signals and vertebrate body axis extension. Development 136: 1591–1604. - PubMed
-
- Tzouanacou E, Wegener A, Wymeersch FJ, Wilson V, Nicolas JF (2009) Redefining the progression of lineage segregations during mammalian embryogenesis by clonal analysis. Dev Cell 17: 365–376. - PubMed
-
- Diez del Corral R, Olivera-Martinez I, Goriely A, Gale E, Maden M, et al. (2003) Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron 40: 65–79. - PubMed
-
- Vermot J, Gallego Llamas J, Fraulob V, Niederreither K, Chambon P, et al. (2005) Retinoic acid controls the bilateral symmetry of somite formation in the mouse embryo. Science 308: 563–566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

