Tonotopic organization of the hyperpolarization-activated current (Ih) in the mammalian medial superior olive
- PMID: 23874271
- PMCID: PMC3708513
- DOI: 10.3389/fncir.2013.00117
Tonotopic organization of the hyperpolarization-activated current (Ih) in the mammalian medial superior olive
Abstract
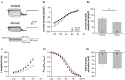
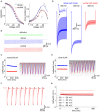
Neuronal membrane properties can largely vary even within distinct morphological cell classes. The mechanisms and functional consequences of this diversity, however, are little explored. In the medial superior olive (MSO), a brainstem nucleus that performs binaural coincidence detection, membrane properties at rest are largely governed by the hyperpolarization-activated inward current (Ih) which enables the temporally precise integration of excitatory and inhibitory inputs. Here, we report that Ih density varies along the putative tonotopic axis of the MSO with Ih being largest in ventral, high-frequency (HF) processing neurons. Also Ih half-maximal activation voltage and time constant are differentially distributed such that Ih of the putative HF processing neurons activate faster and at more depolarized levels. Intracellular application of saturating concentrations of cyclic AMP removed the regional difference in hyperpolarization-activated cyclic nucleotide gated (HCN) channel activation, but not Ih density. Experimental data in conjunction with a computational model suggest that increased Ih levels are helpful in counteracting temporal summation of phase-locked inhibitory inputs which is particularly prominent in HF neurons.
Keywords: HCN channel; coincidence detection; medial superior olive; sound localization; tonotopy.
Figures





References
-
- Adam T. J., Finlayson P. G., Schwarz D. W. F. (2001). Membrane properties of principal neurons of the lateral superior olive. J. Neurophysiol. 86, 922–934 - PubMed
-
- Brandt M. C., Endres-Becker J., Zagidullin N., Motloch L. J., Er F., Rottlaender D., et al. (2009). Effects of KCNE2 on HCN isoforms: distinct modulation of membrane expression and single channel properties. Am. J. Physiol. Heart Circ. Physiol. 297, H355–H363 10.1152/ajpheart.00154.2009 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

