MUC (Memory, Unification, Control) and beyond
- PMID: 23874313
- PMCID: PMC3709422
- DOI: 10.3389/fpsyg.2013.00416
MUC (Memory, Unification, Control) and beyond
Abstract
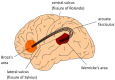
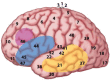
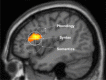
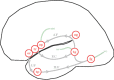
A neurobiological model of language is discussed that overcomes the shortcomings of the classical Wernicke-Lichtheim-Geschwind model. It is based on a subdivision of language processing into three components: Memory, Unification, and Control. The functional components as well as the neurobiological underpinnings of the model are discussed. In addition, the need for extension of the model beyond the classical core regions for language is shown. The attention network and the network for inferential processing are crucial to realize language comprehension beyond single word processing and beyond decoding propositional content. It is shown that this requires the dynamic interaction between multiple brain regions.
Keywords: Control; Memory; Unification; language connectivity; neurobiology of language; speaker meaning.
Figures



References
-
- Baggio G., Hagoort P. (2011). The balance between memory and unification in semantics: a dynamic account of the N400. Lang. Cogn. Process. 26, 1338–1367 10.1080/01690965.2010.542671 - DOI
-
- Baggio G., Van Lambalgen M., Hagoort P. (2008). Computing and recomputing discourse models: an ERP study. J. Mem. Lang. 59, 36–53 10.1016/j.jml.2008.02.005 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

