Desmoglein-2-integrin Beta-8 interaction regulates actin assembly in endothelial cells: deregulation in systemic sclerosis
- PMID: 23874518
- PMCID: PMC3708925
- DOI: 10.1371/journal.pone.0068117
Desmoglein-2-integrin Beta-8 interaction regulates actin assembly in endothelial cells: deregulation in systemic sclerosis
Erratum in
- PLoS One. 2013;8(7). doi:10.1371/annotation/b41766f2-c23d-455e-8d6e-e4bce5ae1d80
Abstract
Background: The inability of endothelial cells of patients affected by the diffuse form of Systemic sclerosis (SSc) to perform angiogenesis is a marker of the disease. We previously demonstrated that desmoglein-2 reduction is a major difference between (SSc)-microvascular endothelial cells (MVECs) and normal (N)-MVECs. Here we investigated the role of desmoglein-2 in human N-MVECs and SSc-MVECs angiogenesis.
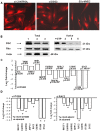
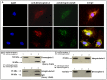
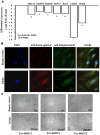
Methodology/principal findings: Angiogenesis was studied by Matrigel invasion, capillary morphogenesis in vitro and Matrigel plug assay in vivo. Gene profiling was studied by Affymetrix technology and signal transduction by Western blotting. Colocalization was validated by immunoprecipitation and confocal microscopy. SiRNAs were used to validate the roles of specific molecules. We observed that desmoglein-2 co-localizes with integrin-beta8 in N-MVECs. This complex is required to signal through Rac, FAK, SMAD1/5 and MAP-kinases, promoting an angiogenic program. Inhibition of desmoglein-2 by DSG2-siRNA impaired actin stress fibres formation, capillary morphogenesis in vitro and angiogenesis in vivo. Transcriptome profiling after DSG2 inhibition revealed alterations of several genes involved in actin organization. siRNA inhibition of integrin-beta8 and RAC2 also resulted into capillary morphogenesis impairment in N-MVECs, due to reduced expression of the same actin-assembly genes that were down-regulated by DSG2 silencing. SSc-MVECs showed down-regulation of the same genes in DSG2-siRNA treated N-MVECs, suggesting that impairment of desmoglein-2/integrin-beta8 complex contributes to angiogenesis derangement in SSc. Transfection of DSG2 in SSc-MVEC partially restored their angiogenic properties in vitro.
Conclusions/significance: We have shown that impairment of actin assembly as a result of desmoglein-2/integrin-beta8 complex formation is a major factor contributing to angiogenesis deregulation in Systemic sclerosis.
Conflict of interest statement
Figures



References
-
- D'Alessio S, Fibbi G, Cinelli M, Guiducci S, Del Rosso A, et al. (2004) Matrix metalloproteinase 12-dependent cleavage of urokinase receptor in systemic sclerosis microvascular endothelial cells results in impaired angiogenesis. Arthritis Rheum 50: 3275–3285. - PubMed
-
- Margheri F, Manetti M, Serratì S, Nosi D, Pucci M, et al. (2006) Domain 1 of the urokinase-type plasminogen activator receptor is required for its morphologic and functional, beta2 integrin-mediated connection with actin cytoskeleton in human microvascular endothelial cells: failure of association in systemic sclerosis endothelial cells. Arthritis Rheum 54: 3926–3398. - PubMed
-
- Giusti B, Serratì S, Margheri F, Papucci L, Rossi L, et al. (2005) The antiangiogenic tissue kallikrein pattern of endothelial cells in systemic sclerosis. Arthritis Rheum 52: 3618–3628. - PubMed
-
- Suda K, Rothen-Rutishauser B, Günthert M, Wunderli-Allenspach H (2001) Phenotypic characterization of human umbilical vein endothelial (ECV304) and urinary carcinoma (T24) cells: endothelial versus epithelial features. In Vitro Cell Dev Biol Anim 37: 505–514. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

