High-content analysis of sequential events during the early phase of influenza A virus infection
- PMID: 23874633
- PMCID: PMC3709902
- DOI: 10.1371/journal.pone.0068450
High-content analysis of sequential events during the early phase of influenza A virus infection
Abstract
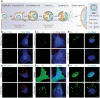
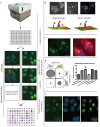
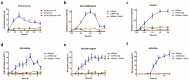
Influenza A virus (IAV) represents a worldwide threat to public health by causing severe morbidity and mortality every year. Due to high mutation rate, new strains of IAV emerge frequently. These IAVs are often drug-resistant and require vaccine reformulation. A promising approach to circumvent this problem is to target host cell determinants crucial for IAV infection, but dispensable for the cell. Several RNAi-based screens have identified about one thousand cellular factors that promote IAV infection. However, systematic analyses to determine their specific functions are lacking. To address this issue, we developed quantitative, imaging-based assays to dissect seven consecutive steps in the early phases of IAV infection in tissue culture cells. The entry steps for which we developed the assays were: virus binding to the cell membrane, endocytosis, exposure to low pH in endocytic vacuoles, acid-activated fusion of viral envelope with the vacuolar membrane, nucleocapsid uncoating in the cytosol, nuclear import of viral ribonucleoproteins, and expression of the viral nucleoprotein. We adapted the assays to automated microscopy and optimized them for high-content screening. To quantify the image data, we performed both single and multi-parametric analyses, in combination with machine learning. By time-course experiments, we determined the optimal time points for each assay. Our quality control experiments showed that the assays were sufficiently robust for high-content analysis. The methods we describe in this study provide a powerful high-throughput platform to understand the host cell processes, which can eventually lead to the discovery of novel anti-pathogen strategies.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

