The anti-cancer property of proteins extracted from Gynura procumbens (Lour.) Merr
- PMID: 23874655
- PMCID: PMC3708952
- DOI: 10.1371/journal.pone.0068524
The anti-cancer property of proteins extracted from Gynura procumbens (Lour.) Merr
Abstract
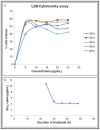
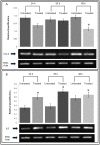
Gynura procumbens (Lour.) Merr. belongs to the Asteraceae Family. The plant is a well-known traditional herb in South East Asia and it is widely used to treat inflammation, kidney discomfort, high cholesterol level, diabetic, cancer and high blood pressure. Our earlier study showed the presence of valuable plant defense proteins, such as peroxidase, thaumatin-like proteins and miraculin in the leaf of G. procumbens. However, the effects of these defense proteins on cancers have never been determined previously. In the present study, we investigated the bioactivity of gel filtration fractionated proteins of G. procumbens leaf extract. The active protein fraction, SN-F11/12, was found to inhibit the growth of a breast cancer cell line, MDA-MB-231, at an EC50 value of 3.8 µg/mL. The mRNA expressions of proliferation markers, Ki67 and PCNA, were reduced significantly in the MDA-MB-23 cells treated with SN-F11/12. The expression of invasion marker, CCL2, was also found reduced in the treated MDA-MB-231 cells. All these findings highlight the anti-cancer property of SN-F11/12, therefore, the proteins in this fraction can be a potential chemotherapeutic agent for breast cancer treatment.
Conflict of interest statement
Figures




References
-
- Tepkeeva II, Moiseeva EV, Chaadaeva AV, Zhavoronkova EV, Kessler YV, et al. (2008) Evaluation of antitumor activity of peptide extracts from medicinal plants on the model of transplanted breast cancer in CBRB-Rb (8.17)1Iem mice. Bulletin of Experimental Biology and Medicine 145: 464–466. - PubMed
-
- Bokesch HR, Pannell LK, Cochran PK, Sowder RC, McKee TC, et al. (2001) A novel anti-HIV macrocyclic peptide from Palicourea condensata . Journal of Natural Products 64: 249–250. - PubMed
-
- Gorjanovic S, Beljanski MV, Gavrovic-Jankulovic M, Gojgic-Cvijovic G, Pavlovic MD, et al. (2007) Antimicrobial Activity of Malting Barley Grain Thaumatin-Like Protein Isoforms, S and R. Journal of the Institute of Brewing. 113: 206–212.
-
- Harvey AL (2008) Natural products in drug discovery. Drug Discovery Today 13: 894–901. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

