Assessment of motor function, sensory motor gating and recognition memory in a novel BACHD transgenic rat model for huntington disease
- PMID: 23874679
- PMCID: PMC3708912
- DOI: 10.1371/journal.pone.0068584
Assessment of motor function, sensory motor gating and recognition memory in a novel BACHD transgenic rat model for huntington disease
Abstract
Rationale: Huntington disease (HD) is frequently first diagnosed by the appearance of motor symptoms; the diagnosis is subsequently confirmed by the presence of expanded CAG repeats (> 35) in the HUNTINGTIN (HTT) gene. A BACHD rat model for HD carrying the human full length mutated HTT with 97 CAG-CAA repeats has been established recently. Behavioral phenotyping of BACHD rats will help to determine the validity of this model and its potential use in preclinical drug discovery studies.
Objectives: The present study seeks to characterize the progressive emergence of motor, sensorimotor and cognitive deficits in BACHD rats.
Materials and methods: Wild type and transgenic rats were tested from 1 till 12 months of age. Motor tests were selected to measure spontaneous locomotor activity (open field) and gait coordination. Sensorimotor gating was assessed in acoustic startle response paradigms and recognition memory was evaluated in an object recognition test.
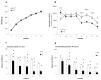
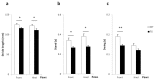
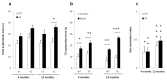
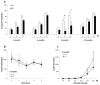
Results: Transgenic rats showed hyperactivity at 1 month and hypoactivity starting at 4 months of age. Motor coordination imbalance in a Rotarod test was present at 2 months and gait abnormalities were seen in a Catwalk test at 12 months. Subtle sensorimotor changes were observed, whereas object recognition was unimpaired in BACHD rats up to 12 months of age.
Conclusion: The current BACHD rat model recapitulates certain symptoms from HD patients, especially the marked motor deficits. A subtle neuropsychological phenotype was found and further studies are needed to fully address the sensorimotor phenotype and the potential use of BACHD rats for drug discovery purposes.
Conflict of interest statement
Figures
References
-
- Douaud G, Gaura V, Ribeiro MJ, Lethimonnier F, Maroy R et al. (2006) Distribution of grey matter atrophy in Huntington’s disease patients: a combined ROI-based and voxel-based morphometric study. NeuroImage 32(4): 1562-1575. doi:10.1016/j.neuroimage.2006.05.057. PubMed: 16875847. - DOI - PubMed
-
- Jones L, Hughes A (2011) Pathogenic mechanisms in Huntington’s disease. Int Rev Neurobiol 98: 373-418. doi:10.1016/B978-0-12-381328-2.00015-8. PubMed: 21907095. - DOI - PubMed
-
- Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED et al. (1985) Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol 44(6): 559-577. doi:10.1097/00005072-198511000-00003. PubMed: 2932539. - DOI - PubMed
-
- Huntington’s Disease Collaborative Research Group (1993) A novel gene containing a trinucleotide repeat that is unstable on Huntington’s disease chromosomes. Cell 72: 971–983. doi:10.1016/0092-8674(93)90585-E. PubMed: 8458085. - DOI - PubMed
-
- Bezprozvanny I, Hayden MR (2004) Deranged neuronal calcium signaling and Huntington disease. Biochem Biophys Res Commun 322(4): 1310-1317. doi:10.1016/j.bbrc.2004.08.035. PubMed: 15336977. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

