Patterned immobilization of antibodies within roll-to-roll hot embossed polymeric microfluidic channels
- PMID: 23874811
- PMCID: PMC3715544
- DOI: 10.1371/journal.pone.0068918
Patterned immobilization of antibodies within roll-to-roll hot embossed polymeric microfluidic channels
Abstract
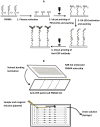
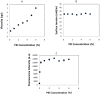
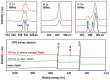
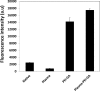
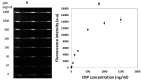
This paper describes a method for the patterned immobilization of capture antibodies into a microfluidic platform fabricated by roll-to-roll (R2R) hot embossing on poly (methyl methacrylate) (PMMA). Covalent attachment of antibodies was achieved by two sequential inkjet printing steps. First, a polyethyleneimine (PEI) layer was deposited onto oxygen plasma activated PMMA foil and further cross-linked with glutaraldehyde (GA) to provide an amine-reactive aldehyde surface (PEI-GA). This step was followed by a second deposition of antibody by overprinting on the PEI-GA patterned PMMA foil. The PEI polymer ink was first formulated to ensure stable drop formation in inkjet printing and the printed films were characterized using atomic force microscopy (AFM) and X-ray photoelectron spectroscopy (XPS). Anti-CRP antibody was patterned on PMMA foil by the developed method and bonded permanently with R2R hot embossed PMMA microchannels by solvent bonding lamination. The functionality of the immobilized antibody inside the microfluidic channel was evaluated by fluorescence-based sandwich immunoassay for detection of C-reactive protein (CRP). The antibody-antigen assay exhibited a good level of linearity over the range of 10 ng/ml to 500 ng/ml (R(2) = 0.991) with a calculated detection limit of 5.2 ng/ml. The developed patterning method is straightforward, rapid and provides a versatile approach for creating multiple protein patterns in a single microfluidic channel for multiplexed immunoassays.
Conflict of interest statement
Figures

Similar articles
-
Surface modification for enhancing antibody binding on polymer-based microfluidic device for enzyme-linked immunosorbent assay.Langmuir. 2006 Oct 24;22(22):9458-67. doi: 10.1021/la061123l. Langmuir. 2006. PMID: 17042569
-
Disposable roll-to-roll hot embossed electrophoresis chip for detection of antibiotic resistance gene mecA in bacteria.Lab Chip. 2012 Jan 21;12(2):333-9. doi: 10.1039/c1lc20782b. Epub 2011 Nov 29. Lab Chip. 2012. PMID: 22127494
-
Protein A-based antibody immobilization onto polymeric microdevices for enhanced sensitivity of enzyme-linked immunosorbent assay.Biotechnol Bioeng. 2009 Feb 15;102(3):891-901. doi: 10.1002/bit.22136. Biotechnol Bioeng. 2009. PMID: 18942150
-
Orientation and characterization of immobilized antibodies for improved immunoassays (Review).Biointerphases. 2017 Mar 16;12(2):02D301. doi: 10.1116/1.4978435. Biointerphases. 2017. PMID: 28301944 Review.
-
Disposable Voltammetric Immunosensors Integrated with Microfluidic Platforms for Biomedical, Agricultural and Food Analyses: A Review.Sensors (Basel). 2018 Nov 24;18(12):4124. doi: 10.3390/s18124124. Sensors (Basel). 2018. PMID: 30477240 Free PMC article. Review.
Cited by
-
Antibodies and antibody-derived analytical biosensors.Essays Biochem. 2016 Jun 30;60(1):9-18. doi: 10.1042/EBC20150002. Essays Biochem. 2016. PMID: 27365031 Free PMC article. Review.
-
Microfluidic-Based Single-Cell Study: Current Status and Future Perspective.Molecules. 2018 Sep 13;23(9):2347. doi: 10.3390/molecules23092347. Molecules. 2018. PMID: 30217082 Free PMC article. Review.
-
Robust Formation of an Epithelial Layer of Human Intestinal Organoids in a Polydimethylsiloxane-Based Gut-on-a-Chip Microdevice.Front Med Technol. 2020 Aug;2:2. doi: 10.3389/fmedt.2020.00002. Epub 2020 Aug 7. Front Med Technol. 2020. PMID: 33532747 Free PMC article.
-
Reducing hepatitis C diagnostic disparities with a fully automated deep learning-enabled microfluidic system for HCV antigen detection.Sci Adv. 2025 Mar 21;11(12):eadt3803. doi: 10.1126/sciadv.adt3803. Epub 2025 Mar 19. Sci Adv. 2025. PMID: 40106555 Free PMC article.
-
Low-cost and cleanroom-free prototyping of microfluidic and electrochemical biosensors: Techniques in fabrication and bioconjugation.Biomicrofluidics. 2021 Nov 8;15(6):061502. doi: 10.1063/5.0071176. eCollection 2021 Dec. Biomicrofluidics. 2021. PMID: 34777677 Free PMC article. Review.
References
-
- St-Louis P (2000) Status of point-of-care testing: promise, realities, and possibilities. Clin. Biochem 33: 427–440. - PubMed
-
- Mark D, Haeberle S, Roth G, von Stetten F, Zengerle R (2010) Microfluidic lab-on-a-chip platforms: requirements, characteristics and applications. Chemical Society Reviews 39: 1153–1182. - PubMed
-
- Von Lode P (2005) Point-of-care immunotesting: Approaching the analytical performance of central laboratory methods. Clin Biochem 38: 591–606. - PubMed
-
- Ramón-Azcón J, Galve R, Sánchez-Baeza F, Marco MP (2005) Development of an Enzyme Linked Immunosorbent Assay for the Determination of the Linear Alkylbenzene Sulfonates and Long-Chain Sulfophenyl Carboxylates Using Antibodies Generated by Pseudoheterologous Immunization. Analytical Chemistry 78: 71–81. - PubMed
-
- Gubala V, Harris LF, Ricco AJ, Tan MX, Williams DE (2011) Point of Care Diagnostics: Status and Future. Analytical Chemistry 84: 487–515. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

