The role of peroxisome proliferator-activated receptor and effects of its agonist, pioglitazone, on a rat model of optic nerve crush: PPARγ in retinal neuroprotection
- PMID: 23874818
- PMCID: PMC3715510
- DOI: 10.1371/journal.pone.0068935
The role of peroxisome proliferator-activated receptor and effects of its agonist, pioglitazone, on a rat model of optic nerve crush: PPARγ in retinal neuroprotection
Abstract
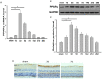
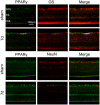
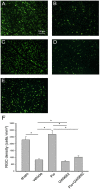
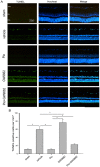
It has been shown that peroxisome proliferators-activated receptor gamma (PPARγ) is beneficial for central nervous system injury. However its role on optic nerve injury remains unknown. In the present study, we examined the change of PPARγ expression in rat retina following optic nerve injury and investigated the effect of pioglitazone (Pio), a PPARγ agonist, on retinal ganglion cells (RGCs) neuroprotection using a rat optic nerve crush (ONC) model. Our results showed that PPARγ mRNA and protein levels were increased after ONC, and most of PPARγ-immunoreactive cells colocalized with Müller cells. Pio treatment significantly enhanced the number of surviving RGCs and inhibited RGCs apoptosis induced by ONC. However, when PPARγ antagonist GW9662 was used, these neuroprotective effects were abolished. In addition, pio attenuated Müller cell activation after ONC. These results indicate that PPARγ appears to protect RGCs from ONC possibly via the reduction of Müller glial activation. It provides evidence that activation of PPARγ may be a potential alternative treatment for RGCs neuroprotection.
Conflict of interest statement
Figures
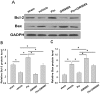
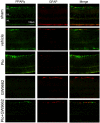
References
-
- Kisiswa L, Dervan AG, Albon J, Morgan JE, Wride MA (2010) Retinal ganglion cell death postponed: giving apoptosis a break? Ophthalmic Res 43: 61–78. - PubMed
-
- Quigley HA, McKinnon SJ, Zack DJ, Pease ME, Kerrigan-Baumrind LA, et al. (2000) Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest Ophthalmol Vis Sci 41: 3460–3466. - PubMed
-
- Sucher NJ, Lipton SA, Dreyer EB (1997) Molecular basis of glutamate toxicity in retinal ganglion cells. Vision Res 37: 3483–3493. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

