Successful human infection with P. falciparum using three aseptic Anopheles stephensi mosquitoes: a new model for controlled human malaria infection
- PMID: 23874828
- PMCID: PMC3712927
- DOI: 10.1371/journal.pone.0068969
Successful human infection with P. falciparum using three aseptic Anopheles stephensi mosquitoes: a new model for controlled human malaria infection
Abstract
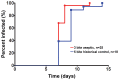
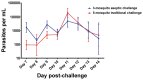
Controlled human malaria infection (CHMI) is a powerful method for assessing the efficacy of anti-malaria vaccines and drugs targeting pre-erythrocytic and erythrocytic stages of the parasite. CHMI has heretofore required the bites of 5 Plasmodium falciparum (Pf) sporozoite (SPZ)-infected mosquitoes to reliably induce Pf malaria. We reported that CHMI using the bites of 3 PfSPZ-infected mosquitoes reared aseptically in compliance with current good manufacturing practices (cGMP) was successful in 6 participants. Here, we report results from a subsequent CHMI study using 3 PfSPZ-infected mosquitoes reared aseptically to validate the initial clinical trial. We also compare results of safety, tolerability, and transmission dynamics in participants undergoing CHMI using 3 PfSPZ-infected mosquitoes reared aseptically to published studies of CHMI using 5 mosquitoes. Nineteen adults aged 18-40 years were bitten by 3 Anopheles stephensi mosquitoes infected with the chloroquine-sensitive NF54 strain of Pf. All 19 participants developed malaria (100%); 12 of 19 (63%) on Day 11. The mean pre-patent period was 258.3 hours (range 210.5-333.8). The geometric mean parasitemia at first diagnosis by microscopy was 9.5 parasites/µL (range 2-44). Quantitative polymerase chain reaction (qPCR) detected parasites an average of 79.8 hours (range 43.8-116.7) before microscopy. The mosquitoes had a geometric mean of 37,894 PfSPZ/mosquito (range 3,500-152,200). Exposure to the bites of 3 aseptically-raised, PfSPZ-infected mosquitoes is a safe, effective procedure for CHMI in malaria-naïve adults. The aseptic model should be considered as a new standard for CHMI trials in non-endemic areas. Microscopy is the gold standard used for the diagnosis of Pf malaria after CHMI, but qPCR identifies parasites earlier. If qPCR continues to be shown to be highly specific, and can be made to be practical, rapid, and standardized, it should be considered as an alternative for diagnosis.
Trial registration: ClinicalTrials.gov NCT00744133 NCT00744133.
Conflict of interest statement
Figures
References
-
- Chulay JD, Schneider I, Cosgriff TM, Hoffman SL, Ballou WR, et al. (1986) Malaria transmitted to humans by mosquitoes infected from cultured Plasmodium falciparum. Am J Trop Med Hyg 35: 66–68. - PubMed
-
- Herrington DA, Clyde DF, Murphy JR, Baqar S, Levine MM, et al. (1988) A model for Plasmodium falciparum sporozoite challenge and very early therapy of parasitaemia for efficacy studies of sporozoite vaccines. Trop Geogr Med 40: 124–127. - PubMed
-
- Ballou WR, Hoffman SL, Sherwood JA, Hollingdale MR, Neva FA, et al. (1987) Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet 1: 1277–1281. - PubMed
-
- Herrington DA, Clyde DF, Losonsky G, Cortesia M, Murphy JR, et al. (1987) Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature 328: 257–259 10.1038/328257a0 [doi]. - DOI - PubMed
-
- Shapiro TA, Ranasinha CD, Kumar N, Barditch-Crovo P (1999) Prophylactic activity of atovaquone against Plasmodium falciparum in humans. Am J Trop Med Hyg 60: 831–836. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

