Platinum-based versus non-platinum-based chemotherapy as first line treatment of inoperable, advanced gastric adenocarcinoma: a meta-analysis
- PMID: 23874831
- PMCID: PMC3708886
- DOI: 10.1371/journal.pone.0068974
Platinum-based versus non-platinum-based chemotherapy as first line treatment of inoperable, advanced gastric adenocarcinoma: a meta-analysis
Abstract
Background: Although the platinum regimen is adopted widely nowadays in spite of the excessive side effects, there is still no international standard for palliative chemotherapy of advanced gastric cancer. This meta-analysis assessed the efficacy and tolerability of platinum versus non-platinum chemotherapy as first-line palliative treatment in patients with inoperable, advanced gastric cancer.
Methods: Randomized phase II and III clinical trials on first-line palliative chemotherapy in inoperable, advanced gastric cancer were identified by electronic searches of PubMed, Embase, and the Cochrane Controlled Trial Register, and hand searches of relevant abstract books and reference lists. Response rates, overall survival, and toxicity were analyzed. Depending on whether new-generation agents (S-1, taxanes and irinotecan) were utilized, the non-platinum regimens were divided into two subgroup.
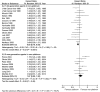
Results: Compared to non-platinum regimens containing new-generation agents, the use of platinum-based regimens was associated with better response (risk ratio (RR) = 1.94, 95%CI[1.48, 2.55], p<0.001), an increase of overall survival (hazard ratio (HR) = 0.85, 95%CI[0.78, 0.92], p<0.001), a higher risk of hematological and non-hematological toxicity. No statistically significant increase in response (RR = 1.03, 95%CI [0.85, 1.24], p = 0.76) or overall survival (HR = 1.07, 95%CI [0.88, 1.30], p = 0.49) was found when platinum therapies were compared to new-generation agent based combination regimens. The toxicity of platinum-based regimens was significantly higher for hematologic toxicity, nausea and vomiting, and neurotoxicity, but not for diarrhea and toxic death rate.
Conclusion: New-generation agent based combination regimens achieved similar response rate and overall survival as platinum-based therapy that had generally higher side effects. S-1, taxanes and irinotecan seemed to be valid options for patients with inoperable, advanced gastric cancer as first-line chemotherapy.
Conflict of interest statement
Figures


Similar articles
-
Platinum-based versus non-platinum-based chemotherapy in advanced non-small-cell lung cancer: a meta-analysis of the published literature.J Clin Oncol. 2005 May 1;23(13):2926-36. doi: 10.1200/JCO.2005.03.045. Epub 2005 Feb 22. J Clin Oncol. 2005. PMID: 15728229
-
Cisplatin versus carboplatin in combination with third-generation drugs for advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2020 Jan 13;1(1):CD009256. doi: 10.1002/14651858.CD009256.pub3. Cochrane Database Syst Rev. 2020. PMID: 31930743 Free PMC article.
-
Irinotecan-involved regimens for advanced gastric cancer: a pooled-analysis of clinical trials.World J Gastroenterol. 2010 Dec 14;16(46):5889-94. doi: 10.3748/wjg.v16.i46.5889. World J Gastroenterol. 2010. PMID: 21155012 Free PMC article.
-
Toward a Treatment Sequencing Strategy: A Systematic Review of Treatment Regimens in Advanced Gastric Cancer/Gastroesophageal Junction Adenocarcinoma.Oncologist. 2021 Oct;26(10):e1704-e1729. doi: 10.1002/onco.13907. Epub 2021 Sep 3. Oncologist. 2021. PMID: 34288262 Free PMC article.
-
Pemetrexed plus platinum as the first-line treatment option for advanced non-small cell lung cancer: a meta-analysis of randomized controlled trials.PLoS One. 2012;7(5):e37229. doi: 10.1371/journal.pone.0037229. Epub 2012 May 17. PLoS One. 2012. PMID: 22615946 Free PMC article. Clinical Trial.
Cited by
-
The role of non-curative surgery in incurable, asymptomatic advanced gastric cancer.PLoS One. 2013 Dec 16;8(12):e83921. doi: 10.1371/journal.pone.0083921. eCollection 2013. PLoS One. 2013. PMID: 24358318 Free PMC article.
-
Quantitative Analysis of HER2 Amplification by Droplet Digital PCR in the Follow-Up of Gastric Cancer Patients Being Treated with Trastuzumab after Surgery.Gastroenterol Res Pract. 2019 Jan 31;2019:1750329. doi: 10.1155/2019/1750329. eCollection 2019. Gastroenterol Res Pract. 2019. PMID: 30838039 Free PMC article.
-
Prognostic value of excision repair cross-complementation group 1 expression in gastric cancer: A meta-analysis.Exp Ther Med. 2015 Apr;9(4):1393-1400. doi: 10.3892/etm.2015.2284. Epub 2015 Feb 11. Exp Ther Med. 2015. PMID: 25780441 Free PMC article.
-
Phase II clinical trial of S-1 plus nanoparticle albumin-bound paclitaxel in untreated patients with metastatic gastric cancer.Cancer Sci. 2018 Nov;109(11):3575-3582. doi: 10.1111/cas.13813. Epub 2018 Oct 26. Cancer Sci. 2018. PMID: 30281875 Free PMC article. Clinical Trial.
-
Non-platinum-based chemotherapy for treatment of advanced gastric cancer: 5-fluorouracil, taxanes, and irinotecan.World J Gastroenterol. 2014 May 14;20(18):5396-402. doi: 10.3748/wjg.v20.i18.5396. World J Gastroenterol. 2014. PMID: 24833869 Free PMC article. Review.
References
-
- Brown LM, Devesa SS (2002) Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am 11: 235–256. - PubMed
-
- Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, et al. (2006) Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 24: 2903–2909. - PubMed
-
- An-ji H (2009) Weekly docetaxel,cisplatin and low dose of fluorouracil for advanced gastric carcinoma:a randomized clinical trial. Tumori 29: 160–163.
-
- Barone C, Corsi DC, Pozzo C, Cassano A, Fontana T, et al. (1998) Treatment of patients with advanced gastric carcinoma with a 5-fluorouracil-based or a cisplatin-based regimen: two parallel randomized phase II studies. Cancer 82: 1460–1467. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

