Platelet lysates produced from expired platelet concentrates support growth and osteogenic differentiation of mesenchymal stem cells
- PMID: 23874839
- PMCID: PMC3708923
- DOI: 10.1371/journal.pone.0068984
Platelet lysates produced from expired platelet concentrates support growth and osteogenic differentiation of mesenchymal stem cells
Abstract
Background: Mesenchymal stem cells are promising candidates in regenerative cell therapy. Conventional culture methods involve the use of animal substances, specifically fetal bovine serum as growth supplement. Since the use of animal-derived products is undesirable for human applications, platelet lysates produced from human platelets are an attractive alternative. This is especially true if platelet lysates from already approved transfusion units at blood banks can be utilized. The purpose of this study was to produce human platelet lysates from expired, blood bank-approved platelet concentrates and evaluate their use as growth supplement in the culture of mesenchymal stem cells.
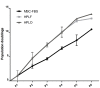
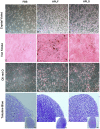
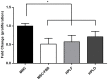
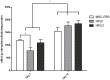
Methodology/principal findings: In this study, bone marrow-derived mesenchymal stem cells were cultured with one of three culture supplements; fetal bovine serum, lysates from freshly prepared human platelet concentrates, or lysates from expired human platelet concentrates. The effects of these platelet-derived culture supplements on basic mesenchymal stem cell characteristics were evaluated. All cultures maintained the typical mesenchymal stem cell surface marker expression, trilineage differentiation potential, and the ability to suppress in vitro immune responses. However, mesenchymal stem cells supplemented with platelet lysates proliferated faster than traditionally cultured cells and increased the expression of the osteogenic marker gene RUNX-2; yet no difference between the use of fresh and expired platelet concentrates was observed.
Conclusion/significance: Our findings suggest that human platelet lysates produced from expired platelet concentrates can be used as an alternative to fetal bovine serum for mesenchymal stem cell culture to the same extent as lysates from fresh platelets.
Conflict of interest statement
Figures
References
-
- Harmening DM, Escobar CE, McGlasson DL (2009) Introduction to hemostasis. In: Harmening DM, editor. Clinical hematology and fundamentals of hemostasis. Philadelphia: F.A. Davis Company. 543–576.
-
- Liles DK, Knupp C (2009) Disorders of Primary Hemostasis: Quantitative and Qualitative Platelet Disorders and Vascular Disorders. In: Harmening DM, editor. Clinical hematology and fundamentals of hemostasis. Philadelphia: F.A. Davis Company. 577–606.
-
- Biousse V (2003) The Coagulation System. J Neuroophthalmol 23: 50–62. - PubMed
-
- Freireich EJ (2011) Origins of platelet transfusion therapy. Transfus Med Rev 25: 252–256. - PubMed
-
- Redler LH, Thompson SA, Hsu SH, Ahmad CS, Levine WN (2011) Platelet-Rich Plasma Therapy: A Systematic Literature Review and Evidence For Clinical Usw. Phys Sportsmed 39: 42–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

