Structural analysis of mitochondrial mutations reveals a role for bigenomic protein interactions in human disease
- PMID: 23874847
- PMCID: PMC3706435
- DOI: 10.1371/journal.pone.0069003
Structural analysis of mitochondrial mutations reveals a role for bigenomic protein interactions in human disease
Abstract
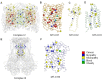
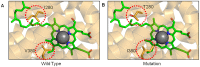
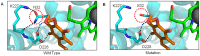
Mitochondria are the energy producing organelles of the cell, and mutations within their genome can cause numerous and often severe human diseases. At the heart of every mitochondrion is a set of five large multi-protein machines collectively known as the mitochondrial respiratory chain (MRC). This cellular machinery is central to several processes important for maintaining homeostasis within cells, including the production of ATP. The MRC is unique due to the bigenomic origin of its interacting proteins, which are encoded in the nucleus and mitochondria. It is this, in combination with the sheer number of protein-protein interactions that occur both within and between the MRC complexes, which makes the prediction of function and pathological outcome from primary sequence mutation data extremely challenging. Here we demonstrate how 3D structural analysis can be employed to predict the functional importance of mutations in mtDNA protein-coding genes. We mined the MITOMAP database and, utilizing the latest structural data, classified mutation sites based on their location within the MRC complexes III and IV. Using this approach, four structural classes of mutation were identified, including one underexplored class that interferes with nuclear-mitochondrial protein interactions. We demonstrate that this class currently eludes existing predictive approaches that do not take into account the quaternary structural organization inherent within and between the MRC complexes. The systematic and detailed structural analysis of disease-associated mutations in the mitochondrial Complex III and IV genes significantly enhances the predictive power of existing approaches and our understanding of how such mutations contribute to various pathologies. Given the general lack of any successful therapeutic approaches for disorders of the MRC, these findings may inform the development of new diagnostic and prognostic biomarkers, as well as new drugs and targets for gene therapy.
Conflict of interest statement
Figures


References
-
- Efremov RG, Sazanov LA (2011) Structure of the membrane domain of respiratory complex I. Nature. 476: 414–420. - PubMed
-
- Junge W, Sielaff H, Engelbrecht S (2009) Torque generation and elastic power transmission in the rotary F(O)F(1)-ATPase. Nature 459: 364–370. - PubMed
-
- Vafai SB, Mootha VK (2012) Mitochondrial disorders as windows into an ancient organelle. Nature 491: 374–383. - PubMed
-
- Hosler J (2012) Biogenesis/assembly of respiratory enzyme complexes. Biochim Biophys Acta 1817: 849–850. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

