The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice
- PMID: 23874851
- PMCID: PMC3712951
- DOI: 10.1371/journal.pone.0069010
The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice
Abstract
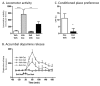
Glucagon-like peptide 1 (GLP-1) is an incretine hormone that controls consummatory behavior and glucose homeostasis. It is released in response to nutrient ingestion from the intestine and production in the brain has also been identified. Given that GLP-1 receptors are expressed in reward areas, such as the nucleus accumbens and ventral tegmental area, and that common mechanisms regulate food and drug-induced reward we hypothesize that GLP-1 receptors are involved in reward regulation. Herein the effect of the GLP-1 receptor agonist Exendin-4 (Ex4), on amphetamine- and cocaine-induced activation of the mesolimbic dopamine system was investigated in mice. In a series of experiments we show that treatment with Ex4, at a dose with no effect per se, reduce amphetamine- as well as cocaine-induced locomotor stimulation, accumbal dopamine release as well as conditioned place preference in mice. Collectively these data propose a role for GLP-1 receptors in regulating drug reward. Moreover, the GLP-1 signaling system may be involved in the development of drug dependence since the rewarding effects of addictive drugs involves interferences with the mesolimbic dopamine system. Given that GLP-1 analogues, such as exenatide and liraglutide, are clinically available for treatment of type II diabetes, we propose that these should be elucidated as treatments of drug dependence.
Conflict of interest statement
Figures

References
-
- Brubaker PL, Anini Y (2003) Direct and indirect mechanisms regulating secretion of glucagon-like peptide-1 and glucagon-like peptide-2. Can J Physiol Pharmacol 81: 1005-1012. doi:10.1139/y03-107. PubMed: 14719035. - DOI - PubMed
-
- Novak U, Wilks A, Buell G, McEwen S (1987) Identical mRNA for preproglucagon in pancreas and gut. Eur J Biochem 164: 553-558. doi:10.1111/j.1432-1033.1987.tb11162.x. PubMed: 3569278. - DOI - PubMed
-
- Merchenthaler I, Lane M, Shughrue P (1999) Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 403: 261-280. doi:10.1002/(SICI)1096-9861(19990111)403:2. PubMed: 9886047. - DOI - PubMed
-
- Holst JJ (2007) The physiology of glucagon-like peptide 1. Physiol Rev 87: 1409-1439. doi:10.1152/physrev.00034.2006. PubMed: 17928588. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

