SWI/SNF enzymes promote SOX10- mediated activation of myelin gene expression
- PMID: 23874858
- PMCID: PMC3712992
- DOI: 10.1371/journal.pone.0069037
SWI/SNF enzymes promote SOX10- mediated activation of myelin gene expression
Abstract
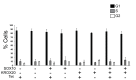
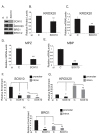
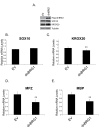
SOX10 is a Sry-related high mobility (HMG)-box transcriptional regulator that promotes differentiation of neural crest precursors into Schwann cells, oligodendrocytes, and melanocytes. Myelin, formed by Schwann cells in the peripheral nervous system, is essential for propagation of nerve impulses. SWI/SNF complexes are ATP dependent chromatin remodeling enzymes that are critical for cellular differentiation. It was recently demonstrated that the BRG1 subunit of SWI/SNF complexes activates SOX10 expression and also interacts with SOX10 to activate expression of OCT6 and KROX20, two transcriptional regulators of Schwann cell differentiation. To determine the requirement for SWI/SNF enzymes in the regulation of genes that encode components of myelin, which are downstream of these transcriptional regulators, we introduced SOX10 into fibroblasts that inducibly express dominant negative versions of the SWI/SNF ATPases, BRM or BRG1. Dominant negative BRM and BRG1 have mutations in the ATP binding site and inhibit gene activation events that require SWI/SNF function. Ectopic expression of SOX10 in cells derived from NIH 3T3 fibroblasts led to the activation of the endogenous Schwann cell specific gene, myelin protein zero (MPZ) and the gene that encodes myelin basic protein (MBP). Thus, SOX10 reprogrammed these cells into myelin gene expressing cells. Ectopic expression of KROX20 was not sufficient for activation of these myelin genes. However, KROX20 together with SOX10 synergistically activated MPZ and MBP expression. Dominant negative BRM and BRG1 abrogated SOX10 mediated activation of MPZ and MBP and synergistic activation of these genes by SOX10 and KROX20. SOX10 was required to recruit BRG1 to the MPZ locus. Similarly, in immortalized Schwann cells, BRG1 recruitment to SOX10 binding sites at the MPZ locus was dependent on SOX10 and expression of dominant negative BRG1 inhibited expression of MPZ and MBP in these cells. Thus, SWI/SNF enzymes cooperate with SOX10 to directly activate genes that encode components of peripheral myelin.
Conflict of interest statement
Figures



Similar articles
-
The microphthalmia-associated transcription factor requires SWI/SNF enzymes to activate melanocyte-specific genes.J Biol Chem. 2006 Jul 21;281(29):20233-41. doi: 10.1074/jbc.M512052200. Epub 2006 Apr 28. J Biol Chem. 2006. PMID: 16648630
-
Activation of Krox20 gene expression by Sox10 in myelinating Schwann cells.J Neurochem. 2010 Feb;112(3):744-54. doi: 10.1111/j.1471-4159.2009.06498.x. Epub 2009 Nov 17. J Neurochem. 2010. PMID: 19922439
-
Heterogeneous SWI/SNF chromatin remodeling complexes promote expression of microphthalmia-associated transcription factor target genes in melanoma.Oncogene. 2010 Jan 7;29(1):81-92. doi: 10.1038/onc.2009.304. Epub 2009 Sep 28. Oncogene. 2010. PMID: 19784067 Free PMC article.
-
The BRG1 ATPase of human SWI/SNF chromatin remodeling enzymes as a driver of cancer.Epigenomics. 2017 Jun;9(6):919-931. doi: 10.2217/epi-2017-0034. Epub 2017 May 19. Epigenomics. 2017. PMID: 28521512 Free PMC article. Review.
-
Pathomechanisms of mutant proteins in Charcot-Marie-Tooth disease.Neuromolecular Med. 2006;8(1-2):217-42. doi: 10.1385/nmm:8:1-2:217. Neuromolecular Med. 2006. PMID: 16775378 Review.
Cited by
-
ATP-dependent chromatin remodeling during mammalian development.Development. 2016 Aug 15;143(16):2882-97. doi: 10.1242/dev.128892. Development. 2016. PMID: 27531948 Free PMC article. Review.
-
Conditional Loss of BAF (mSWI/SNF) Scaffolding Subunits Affects Specification and Proliferation of Oligodendrocyte Precursors in Developing Mouse Forebrain.Front Cell Dev Biol. 2021 Jul 15;9:619538. doi: 10.3389/fcell.2021.619538. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34336815 Free PMC article.
-
Brg1 directly regulates Olig2 transcription and is required for oligodendrocyte progenitor cell specification.Dev Biol. 2016 May 15;413(2):173-87. doi: 10.1016/j.ydbio.2016.04.003. Epub 2016 Apr 8. Dev Biol. 2016. PMID: 27067865 Free PMC article.
-
ACTL6a coordinates axonal caliber recognition and myelination in the peripheral nerve.iScience. 2022 Mar 23;25(4):104132. doi: 10.1016/j.isci.2022.104132. eCollection 2022 Apr 15. iScience. 2022. PMID: 35434551 Free PMC article.
-
Epigenetic modifications-insight into oligodendrocyte lineage progression, regeneration, and disease.FEBS Lett. 2018 Apr;592(7):1063-1078. doi: 10.1002/1873-3468.12999. Epub 2018 Feb 22. FEBS Lett. 2018. PMID: 29427507 Free PMC article. Review.
References
-
- Pereira JA, Lebrun-Julien F, Suter U (2012) Molecular mechanisms regulating myelination in the peripheral nervous system. Trends Neurosci 35: 123-134. doi:10.1016/j.tins.2011.11.006. PubMed: 22192173. - DOI - PubMed
-
- Stys PK, Zamponi GW, van Minnen J, Geurts JJ (2012) Will the real multiple sclerosis please stand up? Nat Rev Neurosci 13: 507-514. doi:10.1038/nrn3275. PubMed: 22714021. - DOI - PubMed
-
- Berger P, Niemann A, Suter U (2006) Schwann cells and the pathogenesis of inherited motor and sensory neuropathies (Charcot-Marie-Tooth disease). Glia 54: 243-257. doi:10.1002/glia.20386. PubMed: 16856148. - DOI - PubMed
-
- Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M et al. (2001) The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev 15: 66-78. doi:10.1101/gad.186601. PubMed: 11156606. - DOI - PMC - PubMed
-
- Zhang SM, Marsh R, Ratner N, Brackenbury R (1995) Myelin glycoprotein P0 is expressed at early stages of chicken and rat embryogenesis. J Neurosci Res 40: 241-250. doi:10.1002/jnr.490400213. PubMed: 7745617. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

