Phosphatidylinositol 4,5-bisphosphate decreases the concentration of Ca2+, phosphatidylserine and diacylglycerol required for protein kinase C α to reach maximum activity
- PMID: 23874859
- PMCID: PMC3707892
- DOI: 10.1371/journal.pone.0069041
Phosphatidylinositol 4,5-bisphosphate decreases the concentration of Ca2+, phosphatidylserine and diacylglycerol required for protein kinase C α to reach maximum activity
Abstract
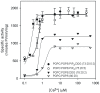
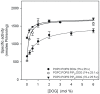
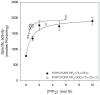
The C2 domain of PKCα possesses two different binding sites, one for Ca(2+) and phosphatidylserine and a second one that binds PIP2 with very high affinity. The enzymatic activity of PKCα was studied by activating it with large unilamellar lipid vesicles, varying the concentration of Ca(2+) and the contents of dioleylglycerol (DOG), phosphatidylinositol 4,5-bisphosphate (PIP2) and phosphadidylserine (POPS) in these model membranes. The results showed that PIP2 increased the Vmax of PKCα and, when the PIP2 concentration was 5 mol% of the total lipid in the membrane, the addition of 2 mol% of DOG did not increase the activity. In addition PIP2 decreases K0.5 of Ca(2+) more than 3-fold, that of DOG almost 5-fold and that of POPS by a half. The K0.5 values of PIP2 amounted to only 0.11 µM in the presence of DOG and 0.39 in its absence, which is within the expected physiological range for the inner monolayer of a mammalian plasma membrane. As a consequence, PKCα may be expected to operate near its maximum capacity even in the absence of a cell signal producing diacylglycerol. Nevertheless, we have shown that the presence of DOG may also help, since the K0.5 for PIP2 notably decreases in its presence. Taken together, these results underline the great importance of PIP2 in the activation of PKCα and demonstrate that in its presence, the most important cell signal for triggering the activity of this enzyme is the increase in the concentration of cytoplasmic Ca(2+).
Conflict of interest statement
Figures

References
-
- Corbalan-Garcia S, Gomez-Fernandez JC (2006) Protein kinase C regulatory domains: the art of decoding many different signals in membranes. Biochim Biophys Acta 1761: 633–654. - PubMed
-
- Nishizuka Y (1995) Protein kinase C and lipid signaling for sustained cellular responses. FASEB J 9: 484–496. - PubMed
-
- Corbalan-Garcia S, Garcia-Garcia J, Rodriguez-Alfaro JA, Gomez-Fernandez JC (2003) A new phosphatidylinositol 4,5-bisphosphate-binding site located in the C2 domain of protein kinase Calpha. J Biol Chem 278: 4972–4980. - PubMed
-
- Guerrero-Valero M, Marin-Vicente C, Gomez-Fernandez JC, Corbalan-Garcia S (2007) The C2 domains of classical PKCs are specific PtdIns(4,5)P2-sensing domains with different affinities for membrane binding. J Mol Biol 371: 608–621. - PubMed
-
- Marin-Vicente C, Nicolas FE, Gomez-Fernandez JC, Corbalan-Garcia S (2008) The PtdIns(4,5)P2 ligand itself influences the localization of PKCalpha in the plasma membrane of intact living cells. J Mol Biol 377: 1038–1052. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

