Antizyme inhibitor 2 hypomorphic mice. New patterns of expression in pancreas and adrenal glands suggest a role in secretory processes
- PMID: 23874910
- PMCID: PMC3709932
- DOI: 10.1371/journal.pone.0069188
Antizyme inhibitor 2 hypomorphic mice. New patterns of expression in pancreas and adrenal glands suggest a role in secretory processes
Abstract
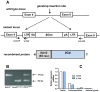
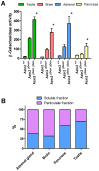
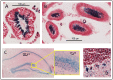
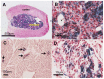
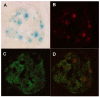
The intracellular levels of polyamines, polycations implicated in proliferation, differentiation and cell survival, are regulated by controlling their biosynthesis, catabolism and transport. Antizymes and antizyme inhibitors are key regulatory proteins of polyamine levels by affecting ornithine decarboxylase, the rate-limiting biosynthetic enzyme, and polyamine uptake. We recently described the molecular function of a novel antizyme inhibitor (AZIN2). However, the physiological function of AZIN2 in mammals is mostly unknown. To gain insight on the tissue expression profile of AZIN2 and to find its possible physiological role, we have generated, transgenic mice with severe Azin2 hypomorphism. This mouse model expresses transgenic bacterial β-D-galactosidase as a reporter gene, under the control of the Azin2 endogenous promoter, what allows a very sensitive and specific detection of the expression of the gene in the different tissues of transgenic mice. The biochemical and histochemical analyses of β-D-galactosidase together with the quantification of Azin2 mRNA levels, corroborated that AZIN2 is mainly expressed in testis and brain, and showed for the first time that AZIN2 is also expressed in the adrenal glands and pancreas. In these tissues, AZIN2 was not expressed in all type of cells, but rather in specific type of cells. Thus, AZIN2 was mainly found in the haploid germinal cells of the testis and in different brain regions such as hippocampus and cerebellum, particularly in specific type of neurons. In the adrenal glands and pancreas, the expression was restricted to the adrenal medulla and to the Langerhans islets, respectively. Interestingly, plasma insulin levels were significantly reduced in the transgenic mice. These results support the idea that AZIN2 may have a role in the modulation of reproductory and secretory functions and that this mouse model might be an interesting tool for the progress of our understanding on the role of AZIN2 and polyamines in specific mammalian cells.
Conflict of interest statement
Figures
Similar articles
-
New insights of polyamine metabolism in testicular physiology: A role of ornithine decarboxylase antizyme inhibitor 2 (AZIN2) in the modulation of testosterone levels and sperm motility.PLoS One. 2018 Dec 19;13(12):e0209202. doi: 10.1371/journal.pone.0209202. eCollection 2018. PLoS One. 2018. PMID: 30566531 Free PMC article.
-
Antizyme inhibitor 2: molecular, cellular and physiological aspects.Amino Acids. 2010 Feb;38(2):603-11. doi: 10.1007/s00726-009-0419-4. Epub 2009 Dec 3. Amino Acids. 2010. PMID: 19956990 Review.
-
A novel role for antizyme inhibitor 2 as a regulator of serotonin and histamine biosynthesis and content in mouse mast cells.Amino Acids. 2016 Oct;48(10):2411-21. doi: 10.1007/s00726-016-2230-3. Epub 2016 Apr 15. Amino Acids. 2016. PMID: 27084713
-
Antizyme inhibitor 2 (AZIN2/ODCp) stimulates polyamine uptake in mammalian cells.J Biol Chem. 2008 Jul 25;283(30):20761-9. doi: 10.1074/jbc.M801024200. Epub 2008 May 28. J Biol Chem. 2008. PMID: 18508777 Free PMC article.
-
Regulation of polyamine biosynthesis by antizyme and some recent developments relating the induction of polyamine biosynthesis to cell growth. Review.Biosci Rep. 1985 Mar;5(3):189-204. doi: 10.1007/BF01119588. Biosci Rep. 1985. PMID: 3893559 Review.
Cited by
-
Structural and degradative aspects of ornithine decarboxylase antizyme inhibitor 2.FEBS Open Bio. 2014 Jun 2;4:510-21. doi: 10.1016/j.fob.2014.05.004. eCollection 2014. FEBS Open Bio. 2014. PMID: 24967154 Free PMC article.
-
A radial histogenetic model of the mouse pallial amygdala.Brain Struct Funct. 2020 Sep;225(7):1921-1956. doi: 10.1007/s00429-020-02097-4. Epub 2020 Jun 24. Brain Struct Funct. 2020. PMID: 32583144 Free PMC article.
-
Molecular Segmentation of the Spinal Trigeminal Nucleus in the Adult Mouse Brain.Front Neuroanat. 2021 Dec 10;15:785840. doi: 10.3389/fnana.2021.785840. eCollection 2021. Front Neuroanat. 2021. PMID: 34955765 Free PMC article.
-
Polyamine biosynthesis in Xenopus laevis: the xlAZIN2/xlODC2 gene encodes a lysine/ornithine decarboxylase.PLoS One. 2019 Sep 11;14(9):e0218500. doi: 10.1371/journal.pone.0218500. eCollection 2019. PLoS One. 2019. PMID: 31509528 Free PMC article.
-
Expression of ODC Antizyme Inhibitor 2 (AZIN2) in Human Secretory Cells and Tissues.PLoS One. 2016 Mar 10;11(3):e0151175. doi: 10.1371/journal.pone.0151175. eCollection 2016. PLoS One. 2016. PMID: 26963840 Free PMC article.
References
-
- Gerner EW, Meyskens FL Jr (2004) Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer 4: 781–792. - PubMed
-
- Pegg AE (2009) Mammalian polyamine metabolism and function. IUBMB Life 61: 880–894 doi:10.1002/iub.230 - DOI - PMC - PubMed
-
- Mangold U (2005) The antizyme family: polyamines and beyond. IUBMB Life 57: 671–676. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

