Serine protease EspP from enterohemorrhagic Escherichia coli is sufficient to induce shiga toxin macropinocytosis in intestinal epithelium
- PMID: 23874912
- PMCID: PMC3715455
- DOI: 10.1371/journal.pone.0069196
Serine protease EspP from enterohemorrhagic Escherichia coli is sufficient to induce shiga toxin macropinocytosis in intestinal epithelium
Abstract
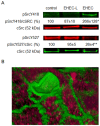
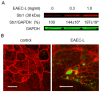
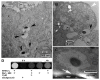
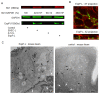
Life-threatening intestinal and systemic effects of the Shiga toxins produced by enterohemorrhagic Escherichia coli (EHEC) require toxin uptake and transcytosis across intestinal epithelial cells. We have recently demonstrated that EHEC infection of intestinal epithelial cells stimulates toxin macropinocytosis, an actin-dependent endocytic pathway. Host actin rearrangement necessary for EHEC attachment to enterocytes is mediated by the type 3 secretion system which functions as a molecular syringe to translocate bacterial effector proteins directly into host cells. Actin-dependent EHEC attachment also requires the outer membrane protein intimin, a major EHEC adhesin. Here, we investigate the role of type 3 secretion in actin turnover occurring during toxin macropinocytosis. Toxin macropinocytosis is independent of EHEC type 3 secretion and intimin attachment. EHEC soluble factors are sufficient to stimulate macropinocytosis and deliver toxin into enterocytes in vitro and in vivo; intact bacteria are not required. Intimin-negative enteroaggregative Escherichia coli (EAEC) O104:H4 robustly stimulate Shiga toxin macropinocytosis into intestinal epithelial cells. The apical macropinosomes formed in intestinal epithelial cells move through the cells and release their cargo at these cells' basolateral sides. Further analysis of EHEC secreted proteins shows that a serine protease EspP alone is able to stimulate host actin remodeling and toxin macropinocytosis. The observation that soluble factors, possibly serine proteases including EspP, from each of two genetically distinct toxin-producing strains, can stimulate Shiga toxin macropinocytosis and transcellular transcytosis alters current ideas concerning mechanisms whereby Shiga toxin interacts with human enterocytes. Mechanisms important for this macropinocytic pathway could suggest new potential therapeutic targets for Shiga toxin-induced disease.
Conflict of interest statement
Figures




References
-
- Serna A 4th, Boedeker EC (2008) Pathogenesis and treatment of Shiga toxin-producing Escherichia coli infections. Curr Opin Gastroenterol 24: 38-47. doi:10.1097/MOG.0b013e3282f2dfb8. PubMed: 18043231. - DOI - PubMed
-
- Trachtman H, Austin C, Lewinski M, Stahl RA (2012) R e n a l a n d n e u r o l o g i c a l i n v o l v e m e n t in t y p i c a l S h i g a t o x i n - a s s o c i a t e d H U S . Nat Rev Nephrol 8(11):658-69 - PubMed
-
- Tarr PI, Gordon CA, Chandler WL (2005) Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 365: 1073-1086. doi:10.1016/S0140-6736(05)74232-X. PubMed: 15781103. - DOI - PubMed
-
- Fuller CA, Pellino CA, Flagler MJ, Strasser JE, Weiss AA (2011) Shiga toxin subtypes display dramatic differences in potency. Infect Immun 79(3): 1329-1337. doi:10.1128/IAI.01182-10. PubMed: 21199911. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

