The synthetic amphipathic peptidomimetic LTX109 is a potent fungicide that disturbs plasma membrane integrity in a sphingolipid dependent manner
- PMID: 23874964
- PMCID: PMC3709891
- DOI: 10.1371/journal.pone.0069483
The synthetic amphipathic peptidomimetic LTX109 is a potent fungicide that disturbs plasma membrane integrity in a sphingolipid dependent manner
Abstract
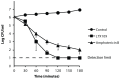
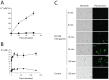
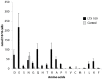
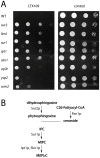
The peptidomimetic LTX109 (arginine-tertbutyl tryptophan-arginine-phenylethan) was previously shown to have antibacterial properties. Here, we investigated the activity of this novel antimicrobial peptidomimetic on the yeast Saccharomyces cerevisiae. We found that LTX109 was an efficient fungicide that killed all viable cells in an exponentially growing population as well as a large proportion of cells in biofilm formed on an abiotic surface. LTX109 had similar killing kinetics to the membrane-permeabilizing fungicide amphotericin B, which led us to investigate the ability of LTX109 to disrupt plasma membrane integrity. S. cerevisiae cells exposed to a high concentration of LTX109 showed rapid release of potassium and amino acids, suggesting that LTX109 acted by destabilizing the plasma membrane. This was supported by the finding that cells were permeable to the fluorescent nucleic acid stain SYTOX Green after a few minutes of LTX109 treatment. We screened a haploid S. cerevisiae gene deletion library for mutants resistant to LTX109 to uncover potential molecular targets. Eight genes conferred LTX109 resistance when deleted and six were involved in the sphingolipid biosynthetic pathway (SUR1, SUR2, SKN1, IPT1, FEN1 and ORM2). The involvement of all of these genes in the biosynthetic pathway for the fungal-specific lipids mannosylinositol phosphorylceramide (MIPC) and mannosyl di-(inositol phosphoryl) ceramide (M(IP)2C) suggested that these lipids were essential for LTX109 sensitivity. Our observations are consistent with a model in which LTX109 kills S. cerevisiae by nonspecific destabilization of the plasma membrane through direct or indirect interaction with the sphingolipids.
Conflict of interest statement
Figures
References
-
- Tortorano AM, Peman J, Bernhardt H, Klingspor L, Kibbler CC, et al. (2004) Epidemiology of candidaemia in Europe: results of 28-month European Confederation of Medical Mycology (ECMM) hospital-based surveillance study. Eur J Clin Microbiol Infect Dis 23: 317–322. - PubMed
-
- Vanden Bossche H, Koymans L, Moereels H (1995) P450 inhibitors of use in medical treatment: focus on mechanisms of action. Pharmacol Ther 67: 79–100. - PubMed
-
- Teerlink T, de Kruijff B, Demel RA (1980) The action of pimaricin, etruscomycin and amphotericin B on liposomes with varying sterol content. Biochim Biophys Acta 599: 484–492. - PubMed
-
- Ermishkin LN, Kasumov KM, Potzeluyev VM (1976) Single ionic channels induced in lipid bilayers by polyene antibiotics amphotericin B and nystatine. Nature 262: 698–699. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

