Age-dependent modulation of synaptic plasticity and insulin mimetic effect of lipoic acid on a mouse model of Alzheimer's disease
- PMID: 23875003
- PMCID: PMC3714252
- DOI: 10.1371/journal.pone.0069830
Age-dependent modulation of synaptic plasticity and insulin mimetic effect of lipoic acid on a mouse model of Alzheimer's disease
Erratum in
- PLoS One. 2014;9(12):e116442
Abstract
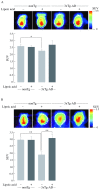
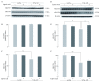
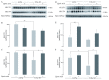
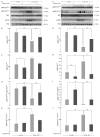
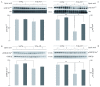
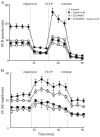
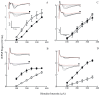
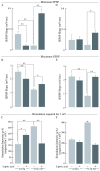
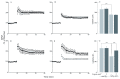
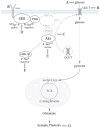
Alzheimer's disease is a progressive neurodegenerative disease that entails impairments of memory, thinking and behavior and culminates into brain atrophy. Impaired glucose uptake (accumulating into energy deficits) and synaptic plasticity have been shown to be affected in the early stages of Alzheimer's disease. This study examines the ability of lipoic acid to increase brain glucose uptake and lead to improvements in synaptic plasticity on a triple transgenic mouse model of Alzheimer's disease (3xTg-AD) that shows progression of pathology as a function of age; two age groups: 6 months (young) and 12 months (old) were used in this study. 3xTg-AD mice fed 0.23% w/v lipoic acid in drinking water for 4 weeks showed an insulin mimetic effect that consisted of increased brain glucose uptake, activation of the insulin receptor substrate and of the PI3K/Akt signaling pathway. Lipoic acid supplementation led to important changes in synaptic function as shown by increased input/output (I/O) and long term potentiation (LTP) (measured by electrophysiology). Lipoic acid was more effective in stimulating an insulin-like effect and reversing the impaired synaptic plasticity in the old mice, wherein the impairment of insulin signaling and synaptic plasticity was more pronounced than those in young mice.
Conflict of interest statement
Figures
Similar articles
-
Effects of Lipoic Acid on High-Fat Diet-Induced Alteration of Synaptic Plasticity and Brain Glucose Metabolism: A PET/CT and 13C-NMR Study.Sci Rep. 2017 Jul 14;7(1):5391. doi: 10.1038/s41598-017-05217-z. Sci Rep. 2017. PMID: 28710347 Free PMC article.
-
Alterations in synaptic plasticity coincide with deficits in spatial working memory in presymptomatic 3xTg-AD mice.Neurobiol Learn Mem. 2015 Nov;125:152-162. doi: 10.1016/j.nlm.2015.09.003. Epub 2015 Sep 15. Neurobiol Learn Mem. 2015. PMID: 26385257 Free PMC article.
-
Blockade of adenosine A2A receptors recovers early deficits of memory and plasticity in the triple transgenic mouse model of Alzheimer's disease.Neurobiol Dis. 2018 Sep;117:72-81. doi: 10.1016/j.nbd.2018.05.024. Epub 2018 May 31. Neurobiol Dis. 2018. PMID: 29859867
-
Synaptic plasticity in animal models of early Alzheimer's disease.Philos Trans R Soc Lond B Biol Sci. 2003 Apr 29;358(1432):821-8. doi: 10.1098/rstb.2002.1240. Philos Trans R Soc Lond B Biol Sci. 2003. PMID: 12740129 Free PMC article. Review.
-
A Novel Therapeutic Approach to Treat Alzheimer's Disease by Neurotrophic Support During the Period of Synaptic Compensation.J Alzheimers Dis. 2018;62(3):1211-1218. doi: 10.3233/JAD-170839. J Alzheimers Dis. 2018. PMID: 29562539 Free PMC article. Review.
Cited by
-
AngIV-Analog Dihexa Rescues Cognitive Impairment and Recovers Memory in the APP/PS1 Mouse via the PI3K/AKT Signaling Pathway.Brain Sci. 2021 Nov 11;11(11):1487. doi: 10.3390/brainsci11111487. Brain Sci. 2021. PMID: 34827486 Free PMC article.
-
Brain and Brown Adipose Tissue Metabolism in Transgenic Tg2576 Mice Models of Alzheimer Disease Assessed Using 18F-FDG PET Imaging.Mol Imaging. 2017 Jan 1;16:1536012117704557. doi: 10.1177/1536012117704557. Mol Imaging. 2017. PMID: 28654383 Free PMC article.
-
Signaling Mechanisms of Selective PPARγ Modulators in Alzheimer's Disease.PPAR Res. 2018 Oct 21;2018:2010675. doi: 10.1155/2018/2010675. eCollection 2018. PPAR Res. 2018. PMID: 30420872 Free PMC article. Review.
-
Exploring the Neuroprotective Potential of Rosiglitazone Embedded Nanocarrier System on Streptozotocin Induced Mice Model of Alzheimer's Disease.Neurotox Res. 2021 Apr;39(2):240-255. doi: 10.1007/s12640-020-00258-1. Epub 2020 Jul 18. Neurotox Res. 2021. PMID: 32683650
-
Neuroimaging of Mouse Models of Alzheimer's Disease.Biomedicines. 2022 Jan 28;10(2):305. doi: 10.3390/biomedicines10020305. Biomedicines. 2022. PMID: 35203515 Free PMC article. Review.
References
-
- Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K et al. (2006) Association between CSF biomarkers and incipient Alzheimer s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol 5: 228-234. - PubMed
-
- Brinton RD (2008) Estrogen regulation of glucose metabolism and mitochondrial function: therapeutic implications for prevention of Alzheimer’s disease. Adv Drug Deliv Rev 60: 1504-1511. doi:10.1016/j.addr.2008.06.003. PubMed: 18647624. - DOI - PMC - PubMed
-
- Swerdlow RH (2011) Brain aging, Alzheimer’s disease, and mitochondria. Biochim Biophys Acta 1812: 1630-1639. doi:10.1016/j.bbadis.2011.08.012. PubMed: 21920438. - DOI - PMC - PubMed
-
- Swerdlow RH, Khan SM (2004) A "mitochondrial cascade hypothesis" for sporadic Alzheimer’s disease. Med Hypotheses 63: 8-20. doi:10.1016/j.mehy.2003.12.045. PubMed: 15193340. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

